MORE THAN TWO CENTURIES OF HUMIC ACID RESEARCH
—WHY SO LONG?
BY MICHAEL SUSIC
RESEARCH SCIENTIST
2 FEBRUARY 2001
(Revised 10 June 2008)
With updates on: 11 July 2010; 18 February 2011; 3 April 2015; 27 October 2015 and 12 January 2018
ABSTRACT
Even though humus was named in 1761 and the extraction of humic acids was reported in 1786, humic acid structure and origin has only recently been determined. Such a time span from the discovery of a substance to the elucidation of its origin and structure is indeed rare, perhaps unique. Yet humic acids are extremely common, and also are precursors to such important natural resources as coal and petroleum. The pressure to improve agriculture in the 18th and 19th centuries was the impetus behind the effort to understand humic acid chemistry, and the growing importance of coal and petroleum in the 20th century further increased this pressure. There has been no shortage of research effort, with about 10,000 reports in the scientific literature. Despite this, the work has been conflicting and confused. There are two principal reasons why progress has been so slow. First, humic acid chemistry was difficult to unravel with the technology that was available before WWII. Second, as a consequence of this, researchers became stubbornly polarized in their interpretation of data and refused to acknowledge valid results that were different to their own. When adequate technology for the study of humic acids became available after WWII, the previous unhealthy attitudes were continued and major errors went unchallenged for long periods of time. It is hoped that with the latest humic acid discoveries a more sensible approach will prevail, and that future progress will be much more rapid.
Introduction
Within the past 150 years or so, the structures of naturally-occurring organic compounds have generally been elucidated within decades of their discovery. A classic example is the structure elucidation of DNA. Even if the exact sequence of amino acids and sugars in for example, a glycoprotein, has not been determined, the number and types of amino acids and sugars is well-known. In contrast, the situation for humic acids has been very different. Humic acids* are brown-black polymeric acids of plant origin that are ubiquitous at the Earth’s surface. They contribute to soil fertility, a fact that has been recognized since ancient times. As the study of humic acids progressed from 1786 when they were first extracted from peat bogs in Germany, their importance has become clearer. Not only are they important for soil fertility, but they are also precursors of kerogens, peat, asphalt, bitumen, petroleum, and coal. But they also have deleterious effects, and in more recent times it has been discovered that humic acids affect the environment by complexing with metals and organics, which can modify the toxicity of heavy metals, pesticides, and herbicides. They can also interfere with the efficiency of industrial processes such as alumina extraction from bauxite, adversely affect water purification, and form carcinogens during raw water disinfection.
Along with a good climate, fertile soils are a necessity for human existence, survival, and enjoyment of life. The cultures of many ancient people were based on an agricultural existence. To them fertile soils were the responsibility of the gods, whom they frequently implored for good crops. As knowledge of Man’s environment slowly grew, especially in the Middle Ages, humans turned their minds to more concrete reasons for good crop yields, rather than the mere whims of mythical gods. Differences in plant litter were a fairly obvious candidate for study, apart from the non-descript entities such as wind, earth, and fire which were in vogue at the time. Hence, the linking of soil fertility with plant litter was no surprise. But the complex nature of this could not have been foreseen. After all, it is now known that plants are composed of a plethora of chemical compounds, and even more are produced in the soil due to microbial alteration of them. Knowledge about this in an era of wind, earth, and fire thinking had to come slowly by observation, experiment, and recording of results. Man’s obvious need for and concern about food, or the scarcity of it, brought humus, and later, humic acids, into sharp focus.
The pressure to improve agriculture in the 18th and 19th centuries was the impetus behind the effort to understand humic acid chemistry. Then, the growing importance of coal and petroleum in the 20th century further increased the pressure. However, from the late 1700s to the mid 1800s researchers were only just discovering such simple things as oxygen, hydrogen, carbon dioxide, and the noble gases, yet the question was being asked:
What are humic acids?
The question came far too early, and with the inadequate knowledge and technology of the time, incorrect viewpoints became stubbornly entrenched. This attitude then persisted even when adequate technology became available after WWII.
* The term humus originally meant all organic compounds of plant origin in the soil, and is used as such in this report. The term humic acids is used for the brown-black, polymeric, alkali-soluble acids found in soils, plants, seagrasses, fungi, sediments, and terrestrial and marine waters.
Plants, Humus, and Humic Acids
Humus is the Latin name for soil, and plant matter in soils was first named as such by Wallerius (1) in 1761. de Saussure (2) introduced it specifically for the dark-colored compounds that later came to be called humic acids. During the period 1630-1750 a search was made for the principle of vegetation, namely, what makes plants grow. Before that time it was known that not all soils had the same fertility, but no-one knew or attempted to find out why this was so. The botanist Linnaeus (1707-1778) classified soils in a manner similar to his classification of plants (refer to Waksman [3]). So when Wallerius defined humus in terms of decomposed plant matter, it was quickly seized upon as the substance that makes plants grow. It was claimed that plants grew by absorbing organic carbon from humus. Surprisingly, this idea persisted until the late 1800s (4,5) despite the fact that de Saussure (2) in 1804 demonstrated that plants derive at least some of their carbon from atmospheric carbon dioxide, and that Leibig (6) in 1841 demonstrated that plants can grow without humus. From 1865 onwards (refer to Waksman [3]) attention was drawn to the role of microorganisms in humus and soil, first by Kette and then by others. Only at this time did the carbon cycle come to be understood. A basic tenet of the carbon cycle is that organic carbon from plants is decomposed by microorganisms to carbon dioxide and returned to the atmosphere, to be reabsorbed by plants. The idea that humic acids per se stimulate plant growth has persisted with the use of commercial humic acid fertilizers and soil conditioners. However, it is now generally believed* that plant hormones, impurities in these preparations, are responsible for increased plant growth. I have shown (unpublished data) that the growth of wheat and tomato seedlings in tap water is not accelerated by the addition of highly purified humic acids. In contrast, I have shown that traces of the steroids pregnanediol and pregnanetriol rapidly accelerate the growth of barley seedlings in tap water. This confirms that trace impurities in humic acid preparations could be responsible for the accelerated plant growth. Although it was originally believed that humic acids directly influenced plant growth by providing a source of carbon, it is now known that the effect, apart from such impurities as growth hormones, is indirect. Humic acids alter soil properties by making them more friable, by buffering pH, by allowing soils to swell with moisture, by complexing with trace metals and making them more readily available to plants, and by releasing bound nutrients such as phosphates from clays. Because of the refractory nature of humic acids, they also control the release rate of carbon to the atmosphere. Soils are a huge buffer of humic acids (7), and since microorganisms decompose them only very slowly (8), soils put a brake on what would otherwise be a rapid return of carbon to the atmosphere. Most other organics such as proteins, lignins, and polysaccharides are recycled far more rapidly.
The earliest studies of humus failed to realize that plants are composed of an enormous number of different organic compounds, and that microorganisms can feed on them and elaborate even more compounds. To elucidate the composition of such a huge mixture would have been a daunting task with the limited technology available in the early 1800s. Fortuitously, humic acids are so refractive (to both chemical and microbial decomposition) that they tend to dominate soil organic matter, which meant that the early workers did not have to be very concerned about separating out other compounds. Initially organic chemistry was poorly understood, but once a basic understanding was established, an explosion in knowledge occurred. This explosion in knowledge about the composition of humus began around 1871 with the discovery of proteins in soils, and continued to around 1920, especially with the work of Schreiner and Shorey (9a, b), who helped to discover such organic components as sterols, hydrocarbons, fatty acids glycerides, resin esters, chitin, cellulose, xylan, sugar alcohols, lecithin, pyridines, amides, amino acids, purine bases, vanillin, numerous aliphatic and aromatic acids, and elemental carbon in humus. Nucleic acids and lignin lagged behind somewhat, and plant hormones a lot more, but by the late 1930s the composition of humus was well known. From when the term humus was coined until its composition was established, about 170 years elapsed, a very long time for a given field of research. But this was only part of the story, because humic acids, the major humus components, defied structural elucidation for much longer. This was not through a lack of effort, though. A review of soil humic acid research was made by Bremner (10) in 1954, and he stated: “The literature concerning the humic fraction of soil organic matter is so extensive that no attempt can be made here to review work carried out on the subject before 1940.” This effort did not abate, and as evidence for this, a search of Biological Abstracts between 1969 and March 1989 (inclusive), gave 3001 reports for the keywords humic substances, humic acids, and fulvic acids. By modelling the data, I estimated that up to 1996 about 7600 reports in European languages, several hundred in Russian, and several in Chinese and Japanese had been published in the scientific literature. Therefore, until now, about 10,000 reports have appeared in the scientific literature. This compares favorably with other very important organic compounds, such as glucose with about 60,000 reports.
* There are some researchers who believe humic acids induce membrane permeability, which allows a more efficient uptake of nutrients and accelerates plant growth. However, for this scenario, humic acids also are not a direct source of carbon.
History of Research
Humic acids were first extracted from peat bogs in Germany by Achard (11) in 1786. They were extracted from plant matter by Vauquelin (12) in 1797, and later from soils by many investigators. de Saussure (2) and Döbereiner (13) began crude studies of humic acids in 1804 and 1822, respectively. The first comprehensive studies were published by Sprengel (14) in 1826. Sprengel extracted humic acids from soil with alkali, the same method that Achard used for peat, and this has been the preferred method ever since for extracting humic acids. As early as 1819 Braconnot (15) added acids to starch and sucrose and formed a dark precipitate that looked like humic acids extracted from soils. This began a major effort to prepare as what was then termed artificial ulmin. Glucose was soon found to give the same type of substances, and by 1835 an explanation for the transformation of carbohydrates to synthetic humic acids was given by Malguti (16). When cellulose was transformed to synthetic humic acids by Mulder (17) in 1839, the genesis of humic acids from polysaccharides was thought to have been confirmed. Only time would tell what a mistake this was because the debate was still raging in 1985, 146 years later! Even though there was agreement about the origin of humic acids at this time, there was much debate about the classification of humic acids. Because humic acids are ubiquitous and can be extracted in different proportions by many solvents, they were extracted from many sources by varying methods. Naturally, the resulting extracts had different solubilities, colors, and textures, the principal properties used to differentiate compounds at the time. This led to the invention of a plethora of new names, confusing their identity, and wasting time on classifications instead of investigating their properties. The multiplicity of names was gradually abandoned, probably because a consensus could not be reached. By the mid 1800s humic acids were commonly characterized by chemical formulas (18,19).
Around the 1870s two discoveries had a major effect on views about humic acids. First, it was demonstrated that other organic compounds with structures as simple as carbon tetrachloride could give dark-colored substances that looked like natural humic acids. Both sugars and these chemicals obviously could not be precursors of humic acids, so the polysaccharide origin of humic acids began to lose favor. Second, the chemical formulas and compositions of humic acids became more and more diverse and confusing, incorporating not only carbon, hydrogen, and oxygen, but also nitrogen, anhydrides, ethers, ketones, hydroxyls, alkyl groups, aromatics, and furans. This complexity, together with the loss of favor of the polysaccharide origin, led to the idea that humic acids were elaborated by microorganisms. Microbiology was a newly discovered and popular field at the time, and was rapidly applied to the humic acid problem, although without any proof. Still, those who favored the microbial transformation of organics argued successfully that such a plethora of elements and groups as had been reported to exist in humic acids could only come from a sort of organic soup, something that could be elaborated by microorganisms. The idea that humic acids come from polysaccharides was strongly revived around 1914 by researchers such as Gortner (20) and Marcusson (21) who were working on coal research. The impetus for this was the discovery that the furan structure was prominent in coal and humic acids. Fischer and Schrader (22), also coal researchers, in 1921 demonstrated that microorganisms rapidly consume polysaccharides, and therefore were adamant that polysaccharides could not be precursors of humic acids. They also claimed that lignin degradation, which was much slower, correlated with the production of humic acids (Note: the correlation was poor). Now that microorganisms were given a raw material, the theory was readily accepted by many researchers, but not all. For example, researchers like Marcusson (23) and Hilpert and Littman (24) vehemently opposed the lignin origin theory. But the lignin origin theory came to predominate, and coal texts such that of Hendricks (25) in 1945 simply repeated the popular concept. Concurrently a protein-lignin origin theory evolved because many researchers found small amounts of nitrogen in humic acids. However, this theory began to wane in the 1950s as more and more evidence accumulated to indicate that the nitrogen was due to proteins loosely complexed to humic acids. In 1938 Waksman (3) wrote a popular book called Humus in which he strongly supported the microbial alteration of lignins as the way in which humic acids were formed. Waksman stated in his book: “No other phase of chemisty has been so much confused as that of humus…” and considered his book to be the first study of humic acids from many different aspects, although he did admit that Wollny did it in 1897 but without the inclusion of microorganisms. Waksman emphasized that the role of bacterial and fungal alteration of plant matter was all-important in humus formation, no doubt because he was a microbiologist. But Bremner (10) in 1954 cautioned against Waksman’s claims, by stating: “Much useful information concerning soil organic matter has been obtained by non-isolative methods of investigation such as Waksman’s system of proximate analysis. It is generally realized, however, that such methods are of very limited and uncertain value, and that to achieve any real progress in the elucidation of the chemical nature of soil organic matter we must return to the isolative method of investigation used by Schreiner and Shorey at the beginning of the century.” (Note: Schreiner and Shorey discovered many components of humus.) This caution went largely unheeded and in the 1950s the transformation of lignins by microorganisms became the dominant theory, and the polysaccharide origin theory was forgotten. Humic acids then came to be seen as some mysterious products derived in an aura of mystery so complex that it could probably never be completely understood. No doubt this led to the fanciful, hypothetical structural drawings of humic acids that began appearing at this time.
The lignin origin theory of necessity gave birth to the concept that humic acids are aromatic rather than aliphatic. This feature created a debate of its own, namely, whether humic acids are aromatic or aliphatic. Many researchers then began looking for just aromatic compounds in humic acids. By the time Waksman wrote his book, the multiple names according to hypothetical classifications had been discarded, as well as the chemical formulas, although Waksman lamented that many chemists still held on to ideas that gave rise to those procedures. The only names that have survived are humus, humic acid, humin, and the term fulvic acid, coined by Oden (26) in 1919. It was not until 1957 that Shapiro (27) again made an attempt to define humic acids by a chemical formula. The disfavor of chemical formulas became so great that I have not seen a single report from the past 60 years, apart from the one by Shapiro, that gave a non-hypothetical chemical formula. The standard procedure has been to quote the carbon, hydrogen, oxygen, and nitrogen (if any) composition of humic acids. The chemical formulas were disdained because the data was so variable and researchers felt that the alteration of lignins by microorganisms was far too complex to be desribed by simple chemical formulas. Shapiro’s work was outstanding because it introduced chromatography, solution-phase IR, and organic solvent extractions into humic acid analysis. However, it was totally ignored, probably because it showed that humic acids, or at least the ethyl acetate soluble fraction, are aliphatic, not aromatic.
In the late 1950s it became popular to use GC and GC-MS as a tool to investigate organic compounds, and humic acids were no exception. Because humic acids are polymers the technique was useless for unaltered humic acids, so they were oxidized according to the much earlier work of Bone et al. (28). Even though GC was popular, the oxidized products were also identified by more conventional methods (29). The products contained mainly aromatic compounds, especially benzene polycarboxylic acids, similar to the mellitic acid that had been obtained by Fischer and Schrader (22) in 1921. Results were interpreted to confirm that humic acids are aromatic compounds, and therefore have a lignin origin. However, the oxidation studies had major flaws which were ignored. Reuter et al. (30) in 1983 demonstrated one of these flaws, namely that the amount of aromatics produced was an artifact of oxidation severity (Note: aromatics can be readily formed from aliphatics such as plant polyketides). The oxidation work had become so entrenched that even in 1989 another report on this oxidation work was published (31). In 1966 a popular book on humic acids was published by Kononova (32), which like Waksman’s book before it, strongly supported the lignin origin theory. In the 1970s KBr IR and solid-state NMR studies became popular, but they demonstrated that humic acids are primarily aliphatics. By the early 1980s many researchers had come to realize that the situation regarding humic acid research had become intolerable. But the problems were viewed differently. One group, mainly supporting the lignin origin theory, helped to form the International Humic Substances Society (IHSS) in 1982. The IHSS was formed to better coordinate humic acid research, especially by collecting a bank of standard humic acid samples so that researchers could work on documented samples in the hope of reducing the huge variability in data. Another group wanted to abandon the lignin origin theory in favor of a theory based on the aliphatic structure of humic acids. For example, in 1985 Farmer and Pisaniello (33) wrote:
“Soil organic matter is by far the most abundant form of organic matter at the Earth’s surface…Yet the nature of its distinctive constituents, the humic substances, remain unknown.”
Waksman had made a similar statement 47 years earlier, so this statement highlighted the lack of progress in humic acid research. Anderson and Russell (34) in 1976 had shown that by polymerizing maleic anhydride, synthetic, aliphatic humic acids were obtained. Farmer and Pisaniello made it quite clear that NMR studies proved that humic acids were aliphatics, not aromatics. However, this evidence did not sway Schnitzer (35), a foremost worker in the humic acid field at the time. He blamed the problem on an incomplete understanding of NMR data. Another popular book on humic acids, Humus Chemistry, had been published by Stevenson (36a) in 1982, again strongly supporting the lignin origin theory of humic acids. Surprisingly, its second edition (36b) of 1994 was not much different. However, more and more researchers in the 1980s and 1990s like Ikan et al. (37) began to report that humic acids contain primarily an aliphatic structure. Also, humic acids of marine origin had been discovered in 1972 by Nissenbaum and Kaplan (38), and were always recognized to be purely aliphatic. Harvey et al. (39) in 1984 even proposed a fatty acid origin for these humic acids. In my studies (40) hydrophobic humic acid derivatives eluted from silica gel gave IR and NMR spectra similar to those of fatty acids, although they were a pale yellow color and gave an intense fluorescence, which distinguished them from fatty acids.
In contrast to the studies of oxidized humic acids, studies of reduced humic acids were few. Interestingly, one study (41) from 1966 found that most of the products were low molecular weight aliphatic compounds, and another (42) from 1987 found that the most prominent structures were due to either 1,4-butanediols or aliphatic ether polymers. With the ever-increasing evidence during the 1980s and 1990s about the aliphatic nature of humic acids, most researchers came to accept that humic acids have at least some aliphatic structure. But the idea of genesis by microorganisms strongly persisted. In stark contrast to this idea, we reported (43) in 1991 that humic acids actually occur in plants, well before plant matter reaches the soil. This was in agreement with a report (44) from 1987 in which humic and fulvic acids were isolated from plant leachates. I also found that humic acids occur in very high concentrations in senescent plant matter that has had no contact with the soil, and in fungi, seagrasses and rotten fruits that do not even contain lignin (40). These results have since been replicated (45,46), and indicate that the microbial origin idea of humic acids is incorrect. In fact, brown leaves, yellow hay, yellow wheat field stubble and burnt toast are all everyday testimony of the in situ chemical conversion of polysaccharides to humic acids. This can be readily demonstrated by extracting the humic acids with alkali, measuring the fluorescence emission and excitation spectra, and comparing them to the spectra of humic acids obtained from soil extracts.
Due to the utter confusion in humic acid research, in a 12-year study using the modern techniques of FT-IR, NMR, GC-MS, HPLC, FT-MS, and fluorescence, I reexamed much of the humic acid research of the past 150 years or so. I discovered that humic acids can be readily synthesized from polysaccharides and sugars, albeit with some reactions that have only become known since the late 1940s. By chromatographing derivatives of synthetic and naturally occurring humic acids on silica gel, I have been able to fractionate them into groups with similar properties. These synthetic and naturally-occuring humic acids have almost identical UV-visible, fluorescence, IR and NMR spectra, together with other similar chemical properties. Many solution IR spectra have very sharp bands, and many NMR spectra have sharp peaks, like one finds in normal IR and NMR organic chemistry, permitting the unequivocal assignment of moieties and structures. This is a summary of the data (40):
1. Microbial alteration of plant organics does not form humic acids. The processes are entirely chemical.
2. Humic acids are entirely aliphatic copolymers of several principal monomeric units originating from polysaccharides. Because there are so many possible monomeric units in the molecule, the variability in composition is enormous. However, the conversion process consists of a small number of well-known reactions to form copolymers of molecular weight near 1000 u.
3. The aliphatic structures readily change to aromatic structures, which however, are not related to lignin-derived compounds such as vanillic and syringic acids.
4. They possess a unique conjugated chelate (keto-enol) moiety that changes the structure of humic acids depending on their environment. This gives them almost infinite variety, no doubt contributing to the mystery that has surrounded them.
5. They stick to almost any substance, including large proteins, minerals, and soil particles. Because of this they can as an example solubilize 10x their own weight of clay particles. If they are not rigorously separated from these particles, they can appear to be extremely large molecules.
6. They degrade readily by well-known chemical pathways (e.g. decarboxylation) to form kerogen, bitumen, asphalt, shale oil, petroleum, and coal tar, the exact conversion probably depending on time and external conditions in the natural environment.
7. Their natural conversions can be readily mimicked and altered in the laboratory, but with greater control. Therefore new compounds unknown in nature but related to components of kerogen, bitumen, asphalt, shale oil, and petroleum can be synthesized in the laboratory.
Lack of Technology
In the late 1800s when it was discovered that compounds other than sugars such as carbon tetrachloride give dark-colored products, the nature of the products could not be distinguished due to a lack of technology. At the time solubilities, colors, textures and degradation of molecules were the principal analytical tools, quite inadequate for distinguishing any differences in the dark-colored products. When I repeated the reactions with polysaccharides, sugars, acetone, and acetic anhydride, and analyzed the products by FT-IR, NMR, and GC-MS, differences in the products were obvious. Some sugars did indeed form humic acids, others did not, and the chemicals such as acetone and acetic anhydride formed very different polymers. Therefore, with adequate technology, there would have been no valid reason to dismiss the polysaccharide origin of humic acids as occurred in the late 1800s. Also, in the late 1800s humic acids were found to contain a plethora of moieties, which indicated to researchers at the time that humic acids must have a far more complex origin than simply polysaccharides. However, humic acids are just one component of humus which contains many organic compounds. Without adequate separation methods in the late 1800s, the humic acid preparations would have been impure and contained many other organic compounds. Humic acids per se may not have contained the many elements and moieties that were found. Therefore, the speculation that microorganisms must produce humic acids because of their complexity would have been unfounded with the help of appropriate technology. Only after WWII were such tools as chromatography, IR, NMR, GC, and MS widely introduced in analytical chemistry. For humic acid studies these tools were indispensable. Prior to this time structural elucidations were painstakingly achieved by disassembling molecules piece by piece using chemical reactions. Then the molecule was synthesized and its properties compared to the natural compound. This approach was useless for humic acids because the only way that the molecule could be disassembled was by oxidation, which destroys the entire molecule and the products are mainly carbon dioxide with some oxalic, succinic, and maleic acids, various keto acids and minor amounts of vanillic and syringic acids if the sample has a source containing lignins. The situation is further complicated because benzene polycarboxylic acids are formed as artifacts of the reaction. So all of this information was useless for structural elucidation.
There can be little doubt that before WWII the technology for the structural elucidation of humic acids simply did not exist. But with the huge amount of work that had been done, the foundation should have been set to resolve the matter quickly after WWII with the advent of adequate technology. However, this was not the case because in 1985 researchers were still arguing about such basic matters as whether humic acids were aromatic or aliphatic! The next section investigates the reasons for this.
Unchallenged Errors
Fischer and Schrader (22) claimed that humic acid formation correlated with lignin degradation, and this gave many researchers confidence that their speculation about microorganisms mediating humic acid formation was correct. However, even if a correlation is high, and Fischer and Schrader’s certainly was not, it does not prove a process. In this case it would still have been necessary to actually feed lignins to microorganisms and measure the humic acids, if any, produced. This experiment was NEVER DONE, or at least never reported. Yet without it the microbial alteration of lignins is simply speculation. Then to make matters worse, Waksman (3) claimed that microbiology alone could determine humic acid composition. No doubt this ridiculous overstatement was simply his biased opinion as a microbiologist. But the unfortunate part is that his errors were not properly challenged. Bremner (10) did it 16 years later, albeit not very strongly, but contemporaries were silent. This meant that by the 1950s the belief in the microbial conversion of plant lignins had an almost religious awe to it. Marcusson (23) in 1925 had extracted humic acids from Sphagnum peat, a source that he correctly identified as having very little lignin (47). To have any credence the lignin origin theory must explain this discrepency, but it has simply been ignored. Further, Grosskopf (48) in 1929 had found humus-like substances forming at cellulose (Note: not lignin) decomposition sites on the cell walls of conifer needles. Again, the evidence was ignored.
Another mandatory experiment was also NOT DONE. The microbial alteration of organics to form humic acids would be meaningless if the humic acids already existed before the plant matter reached the soil. Vauquelin (12) in 1797 had extracted humic acids from plant matter, so why was this not checked? We have shown (43) that there is an abundance of humic acids in plant matter, and that the humic acids are leached into the soil from the senescent plant matter by rainfall. One can see this phenomenon every day with leaves lying in puddles of water after heavy rains, and the water becoming reddish-brown in color. Frimmel and Bauer (44) in 1987 inadvertently did this experiment in the laboratory, and it has been done frequently since (45,46), proving that the role of soil microorganisms in humic acid formation has been a red-herring. Yet, these simple experiment were not done until the 1990s!
Shapiro (27) in 1957 made major advances in humic acid research by introducing chromatography, solution IR, and organic solvents. He clearly showed that humic acids, or at least the ethyl acetate-soluble humic acids, are aliphatic. Why were his methods not adopted, and why was his work totally ignored? When I reexamined his work, I found that solution IR was far superior to any other analytical technique, yet no researcher had adopted this technique! However, his contemporaries spent much time trying to find aromatics by the oxidation method, so one can only conclude that the researchers were solely trying to prove a preconceived idea, no more. If the oxidation method had been useful, one could be a little less harsh in criticizing this work, but it had three major flaws. First, most of the molecule was destroyed by the oxidation process, so attention was directed to the 2-5% (in rare cases more) of material that remained, which was mainly aromatic. The best scenario that could be deduced from such a wholesale destruction of the molecule is that aromatics are less susceptible to oxidation than aliphatics. Second, aliphatics, especially polyketide-type structures, can form aromatics readily, and Reuter et al. (30) quite clearly showed that the aromatics in these experiments were artifacts of the process. Third, the aromatics that were obtained in the greatest amount were benzene polycarboxylic acids, compounds that are not lignin degradation products. Lignin degradation products such as vanillic and syringic acids were obtained in trace amounts, but these compounds are ubiquitous and could have been contaminants. These serious errors were challenged in a small way by Reuter et al. (30), but many years after this method was at its peak of popularity. Why was it not challenged earlier, and strongly, because of its serious flaws?
Solid-state NMR studies in the 1970s and 1980s, although lacking sharp peaks, clearly showed humic acids to be mainly aliphatic. The highest aromaticity obtained was 20-40% (37). When it was made known by Farmer and Pisaniello (33) in 1985 that humic acids are aliphatic, and that aromatic moieties are probably contaminants, Schnitzer (34) continued to defend the aromatic structure and lignin origin theory. NMR had been the technique of choice for structure elucidation of organic compounds, both big and small, for around three decades, yet amazingly Schnitzer blamed the discrepency between NMR data and the supposed aromaticity of humic acids on a poor understanding of NMR data! Surely the obvious conclusion should have been that humic acids simply are not aromatic compounds, or at least that they are principally aliphatic. A report by Schulten and Schnitzer (49) in 1993 certainly showed far more aliphatic content than was previously accepted, but the hypothetical humic acid structure was based only on evidence obtained by Schnitzer and colleagues. Farmer and Pisaniello had earlier made the criticism that aromatic structural evidence seemed to come only from laboratories that Schnitzer was associated with. Why was this ignored and once again a highly aromatic structure presented?
Popular books about humic acids were written by Waksman (3) in 1938, Kononova (32) in 1966, and Stevenson (36a) in 1982. Each one strongly supported the aromaticity and lignin origin theory of humic acids. This had a strong influence on humic acid research, and because the authors refused to address the evidence in favor of the polysaccharide origin of humic acids, probably many researchers were discouraged in the past 70 years from pursuing this line of research. The 2nd edition of Stevenson’s book (36b) in 1994, though acknowledging that there is a polysaccharide pathway (because marine humic acids are clearly aliphatic), still promoted the aromatic character and lignin origin theory of humic acids. However, now it was even more complicated in the guise of the polyphenol theory. Surely by now the time had come to give equal space to the evidence that indicated the entire theory was wrong! As the Humic Acids Research Group states on its web site (50), “Without structural knowledge of humic substances, we will continue to rattle around in the black box of ignorance when asked to predict and forecast the impacts of chemical and biological actions on our environment.” One of the main reasons that we have been “rattling around in the black box” for so long is that major errors went unchallenged far too long. In 2003 a book written by KH Tan (51) made a significant step forward by discussing the carbohydrate origin theory of humic acids in detail. Tan correctly pointed out that humic acids are formed in environments that lack lignin, so this theory obviously needs attention. However, the carbohydrate origin is proposed to occur via Maillard condensation, and Tan believed that sugars could escape rapid microbial assimilation by “hiding” in soil interstices. In my personal discussions with him, he was unaware of the xylan (hemicellulose) origin of humic acids (via rapid biochemical conversion of xylose to furfural followed by oxidation to 4-oxobutenoic acid that then polymerized to humic acids) at the time that he wrote his book, but was favorable to the idea after becoming aware of it.
Why so Long?
The search for humic acid structure and origin has taken so long for two major reasons. Because of the pressure to provide adequate food, the question: What are humic acids? was asked far too early. Further pressure came from the discovery that humic acids are precursors of such important natural resources as coal and petroleum. Before WWII the technology simply was not available to answer this question satisfactorily. However, after WWII, with the advent of chromatography, IR, GC, NMR, and MS, it should not have taken long to answer the question. After all, a huge amount of work had been done to lay the foundation of humic acid research. The other reason for the tardiness of humic acid research has been the failure of the scientific method to curb speculation. Major errors went unchallenged completely or at least partially for long periods of time. Researchers refused to address alternative evidence that contradicted their own views, debates became acrimonious, and opinions polarized. Due to the polarization of opinions, strong evidence for the polysaccharide origin of humic acids was not even mentioned in textbooks, yet major flaws in the research promoting the lignin origin theory were ignored.
In conclusion one must strongly speculate that researchers in the field of humic acids have been overly obsessed with the need to consolidate careers, defeat competitors, and save face with colleagues. After all, why care about the exact structure of humic acids if you lose your colleagues and/or job in the process?
References
1. Wallerius, J.G. De Humo (Diss. Upsala, Sweden) Agriculturae fundamenta chemica spez. (1761).
2. de Saussure, T. Recherches chimiques sur la vegetation (Paris, France) (1804).
3. Waksman, S.A. Humus, 2nd ed. (Bailliere, Tindall &Cox, London, UK, 1938).
4. Grandeau, L. Recherches sur le role des matieres organiques du sol dans les phenomenes del nutrition des vegetaux (Nancy, France) (1872).
5. Johnson, S.W. How crops feed. A treatise on the atmosphere and the soil (Orange Judd, New York, USA) (1883).
6. Leibig, J. v. Organic chemistry in its applications to agriculture and physiology, Trans. Webster, J.W. and Owen, J. (Cambridge, UK) (1841).
7. Susic, M. and Isdale, P. Hydraulic and Environmental Modelling of Coastal, Estuarine and River Waters (eds. Falconer, R.A., Goodwin, P. and Matthews, R.G.S.) 588-597 (Aldershot, UK, 1989).
8. Moran, M.A. and Hodson, R.E. Limnol. Oceanogr. 35, 1744 (1990).
9. Schreiner, O. and Shorey, E.C. (a) J. Am. Chem. Soc. 30, 1599-1607 (1908), (b) J. Biol. Chem. 8, 385-393 (1910).
10. Bremner, J.M. J. Soil Sci. 5, 214-232 (1954).
11. Achard, F.K. Crell’s Chem. Ann. 2, 391-403 (1786).
12. Vauquelin, C. Ann. Chim. 21, 39-47 (1797).
13. Döbereiner, Phytochemie 1822, 64 (1822).
14. Sprengel, C. Kastener’s Arch. Ges. Naturelehre 8, 145 (1826).
15. Braconnot, H. Ann. Chim. Phys. 12, 172-195 (1819).
16. Malguti, M. ibid., 59, 407-423 (1835).
17. Mulder, G.J. J. prakt. Chem. 16, 495-497 (1839).
18. Berzelius, J.J. Kgl. Svenska Vet. Akad. (Stockholm,Sweden) (1883).
19. Hermann, R. J. prakt. Chem. 27, 165-172 (1842).
20. Gortner, R.A. J. Biol. Chem. 26, 177-204 (1916).
21. Marcusson, J. Chem. Ztg. 44, 43 (1920).
22. Fischer, F. and Schrader, H. Brennstoff-Chem. 2, 37-45 (1921).
23. Marcusson, J. Z. angew. Chem. 38, 339-341 (1925).
24. Hilpert, R.S. and Littman, E. Ber. deut. chem. Ges. 67B, 1551-1556 (1934).
25. Hendricks, T.A. in Chemistry of Coal Utilization (ed. Lowry, H.H.) 1-24 (Wiley, New York, USA, 1945).
26. Odén, S. Kolloidchem. Beih. 11, 75 (1919).
27. Shapiro, J. Limnol. Oceanogr. 2, 161-179 (1957).
28. Bone, W.A., Parsons, L.G.B., Sapiro, R.H. and Groocock, C.M. Proc. Roy. Soc. A, 148, 492-522 (1934).
29. Wright, J.R. and Schnitzer, M. Can. J. Soil Sci. 39, 44-53 (1958).
30. Reuter, J.H., Ghosal, M., Chian, E.S.K. and Giabbai, M. in Aquatic and Terrestrial Humic Materials (eds. Christman, R.F. and Gjessing, E.T.) 107-125 (Ann Arbor Science, Ann Arbor, USA, 1983).
31. Griffith, S.M. and Schnitzer, M. in Humic Substances II (eds. Hayes, M.H.B., MacCarthy, P., Malcolm, R.L. and Swift, R.S.) 69-98 (Wiley-Interscience, Chichester, USA, 1989).
32. Kononova, M.M. Soil Organic Matter, 2nd Eng. ed., Trans. Nowakowski, T.Z. and Newman, A.C.D. (Pergamon, Oxford, UK, 1966).
33. Farmer, V.C. and Pisaniello, D.L. Nature 313, 474-475 (1985).
34. Anderson, H.A. and Russell, J.D. ibid., 260, 597 (1976).
35. Schnitzer, M. ibid., 316, 658 (1985).
36. Stevenson, F.J. Humus Chemistry (a) (John Wiley , New York, USA, 1982), (b) 2nd ed. (John Wiley, New York, USA, 1994).
37. Ikan, R., Ioselis, P., Rubinsztain, Y., Aizenshtat, Z., Pugmire, R., Anderson, L.L. and Ishiwatari, R. Naturwiss. 73, 150-151 (1986).
38. Nissenbaum, A. and Kaplan, I.R. Limnol. Oceanogr. 17, 570-582 (1972).
39. Harvey, G.R., Boran, D.A., Piotrowicz, S.R. and Weisel, C.P. Nature 309, 244-246 (1984).
40. Susic, M. Structure and origin of humic acids and their relationship to kerogen, bitumen, petroleum and coal. At http:/humicacid.wordpress.com
41. Mendez, J. and Stevenson, F.J. Soil Sci. 102, 85-93 (1966).
42. Martin, F., González-Vila, F.J. and Almendros, G. The Science of the Total Environment 62, 121-128 (1987).
43. Susic, M., Boto, K. and Isdale, P. Mar. Chem. 33, 91-104 (1991).
44. Frimmel, F.H. and Bauer, H. The Science of the Total Environment 62, 139-148 (1987).
45. Davies, G., Fataftah, A., Ghabbour, E.A., Jansen, S.A., Radwan, A. and Raffauf, R.F. Sci. Total Environ. 201, 79-87 (1997).
46. Davies, G., Fataftah, A., Radwin, A., Willey, R.J., Ghabbour, E.A. and Jansen, S.A. in The Role of Humic Substances in the Ecosystems and in Environmental Protection, (eds. Drozd, J., Gonet, S.S., Senesi, N. and Weber, J.), 567-572 (Polish Society of Humic Sustances, Wroclaw, Poland, 1997/8).
47. Hänninen, K.I. The Science of the Total Environment 62, 193-200 (1987), and references therein.
48. Grosskopf, W. Brennstoff-Chemie 10, 293 (1929).
49. Schulten, H.-R. and Schnitzer, M. Naturwiss. 80, 29-30 (1993).
50. At http://www.hagroup.neu.edu
51. Tan, K. H. Humic Matter in Soil and the Environment Principles and Controversies (CRC Press, Boca Raton, USA, 2003).
THE CRITICAL EXPERIMENT THAT WAS NEVER DONE
18 February 2011
The structure of humic acids has always been in dispute. However, the origin and genesis of humic acids has had much less controversy. The reason for this is that a critical experiment was never done or at least never reported until we did this in 1989 and 1991 (Susic, M. & Boto, K.G. J. Chromatogr. 482, 175-187 (1989); Susic, M., Boto, K. and Isdale, P. Mar. Chem. 33, 91-104 (1991)), and by that time the theory of microbial conversion of plant matter in the soil developed in the 1920s and 1930s had been almost universally accepted. In the above studies we demonstrated that humic acids per se were already present in plant matter before it even reached the soil. Later research showed that humic acids were also present in fungi and sea grasses. The 1991 research report stated: “Humic acid persists throughout the lifespan of a leaf but is lost to the soil when the leaf decomposes.”
Why this critical experiment was never done or reported is an enigma, and has resulted in total ignorance regarding the origin and genesis of humic acids. Therefore, I recently revisited this matter in detail and have shown conclusively that senescent plant matter contains enormous amounts of humic acids that are readily extracted and have fluorescence emission spectra consistent with humic acids extracted from soils and Leonardite coal deposits.
Commercially available sugar cane mulch was extracted, filtered and purified as shown in the following photos:
 |
 |
| Extracting dry, senescent sugar cane mulch with 0.1 M KOH |
The extract was filtered, centrifuged and then acidified |
Note that not all of the humic acid can be extracted with alkali because much seems to bind to proteins or other organic matter. Therefore, an estimate of 3% humic acids in senescent plant matter is not unrealistic.
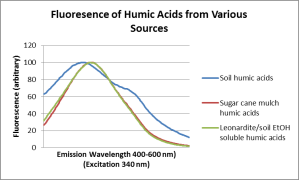
Fluorescence spectroscopy is a powerful tool to compare humic acids, and this technique was used to compare the purified humic acids extracted from sugar cane mulch with humic acids from other sources. The scans are reproduced here:

These series of fluorescence scans demonstrate the similarity of alkali soluble humic acid fractions derived from soil, Leonardite coal deposits and kelp.
From these scans a small difference between the alkali soluble humic acids (soil) and ethanol soluble humic acids (Leonardite/soil) can be observed. The alkali soluble sugar cane mulch humic acids fluorescence exactly matches the ethanol soluble (Leonardite/soil) humic acids.
The fact that humic acids are generated in senescent plant matter is always before our very eyes, yet this was missed for about 200 years! Anywhere in nature where there is a black, brown or yellow color, often it is from humic acids. From the yellow fields of ripe wheat in Australia to the yellow fields of ripe corn in the USA to the dark Negro River flowing into the lighter Amazon river in Brazil, these colors are due to humic acids. The photos below show the ubiquitous presence of humic acids in the environment. The plant matter can be readily extracted with alkali and purified, and fluorescence spectra are similar or identical to those obtained from humic acids extracted from soils.
 |
 |
 |
 |
 |
 |
 |
 |
COMMERCIAL HUMATES—BENEFICIAL OR SCAM?
12 January 2018
The peer-reviewed international journal Agronomy 2016, 6(4), 50 (http://www.mdpi.com/2073-4395/6/4/50/htm) published a Review on 28 October 2016 by Drs Graham Lyons and Yusuf Genc entitled “Commercial Humates in Agriculture: Real Substance or Smoke and Mirrors?”, and this is the abstract:
Soil humic substances (HS) are known to be beneficial for soils and plants, and most published studies of HS and humates, usually conducted under controlled conditions, show benefits. However, the value of commercial humate application in the field is less certain. This review attempts to answer the question: How effective are commercial humates in the field? Commercial humates, especially K humate, are used widely in agriculture today as “soil conditioners”. A wide range of benefits is claimed, including growth of beneficial soil microbes; deactivation of toxic metals; improvements in soil structure including water retention capacity, enhanced nutrient and micronutrient uptake and photosynthesis; resistance to abiotic stress, including salinity; and increased growth, yield and product quality. Despite this, there is a surprising lack of solid evidence for their on-farm effectiveness and findings are often inconsistent. The industry relies largely on anecdotal case studies to promote humates, which are often applied at unrealistically low levels. It is recommended that products should be well characterised, physically and chemically, and that careful field studies be conducted on foliar humate application and pelletised humates at realistic rates, targeted to the seedling rhizosphere, for a variety of crops in a range of soils, including low C sandy and saline soils.
These are some quotes from the Review:
“A lack of solid evidence has not inhibited enthusiastic promotion of humate products by some businesses, and “unfortunately, many of the small (fertiliser) companies make unsubstantiated and often ridiculous claims about humic (and fulvic) acids, which gives these products a poor image when the supposed results do not eventuate.””
The authors refer to an article written by Dr D.C. Edmeades from New Zealand (http://agknowledge.co.nz/uploads/fert-review/Fertiliser-Review-Issue-25.pdf). This is a must read article and I give two quotes here:
“Having unjustly put the fear-of-god into the farmer’s mind, the next step in their advertising is to present their “solution.””
“Graeme Sait of Nutri-Tech Solutions says: “Humates are now recognized as the single most productive input in sustainable agriculture.””
The authors of the Agronomy Review conclude “that the smoke and mirrors description of commercial humates is currently more appropriate than real substance.”, i.e. commercial humates are basically a waste of money for on-farm use.
Why is the situation like this? The authors explain: “Vendors know they will not be prosecuted, regardless of their claims (bold type mine), and researchers have called for mandatory quality control by regulatory bodies.” In other words, government regulators such as Departments of Agriculture are failing to take action against the exaggerated and ludicrous claims being made for commercial humates by companies selling these products. Therefore, BUYER BEWARE!
Does this mean that soils cannot be improved by the addition or replenishment of humates? Not at all! The journal Agronomy 2016, 6, 45 (http://www.mdpi.com/2073-4395/6/4/45) on 28 September 2016 published my communication entitled “Replenishing Humic Acids in Agricultural Soils”, and this is the abstract:
For many decades, it was commonly believed that humic acids were formed in soils by the microbial conversion of plant lignins. However, an experiment to test whether these humic acids were formed prior to plant matter reaching the soil was never reported until the late 1980s (and then only as a side issue), even though humic acids were first isolated and reported in 1786. This was a serious omission, and led to a poor understanding of how the humic acid content of soils could be maintained or increased for optimum fertility. In this study, commercial sugar cane mulch and kelp extracts were extracted with alkali and analyzed for humic acid content. Humic acids in the extracts were positively identified by fluorescence spectrophotometry, and this demonstrated that humic acids are formed in senescent plant and algal matter before they reach the soil, where they are then strongly bound to the soil and are also resistant to microbial metabolism. Humic acids are removed from soils by wind and water erosion, and by water leaching, which means that they must be regularly replenished. This study shows that soils can be replenished or fortified with humic acids simply by recycling plant and algal matter, or by adding outside sources of decomposed plant or algal matter such as composts, mulch, peat, and lignite coals.
My long-time research in the field of humic acids has shown that it is vital to replenish soil humic acids content, and the best ways to do this is by recycling plant matter or to add outside sources of decomposed plant or algal matter such as composts, mulch, peat, seaweed and lignite coals. An added benefit to this approach is the low cost compared to commercial humates.
GIRL OR BOY? NEW FULVIC ACIDS RESEARCH
3 April 2015
Early in my career I read a claim about an ancient Egyptian belief. These ancient people were thought to believe that if the urine of a pregnant woman increased the growth of wheat she would have a girl, and if it increased the growth of barley she would have a boy.
I tested this concept with diluted urine (so that the minerals would not affect plant growth) and found that it was not true for modern wheat and barley, and probably also not true for ancient wheat and barley. However, what I discovered was that two minor hormones in the urine of a pregnant woman (pregnanetriol and pregnenolone) increased the growth of barley by a substantial amount of about 10%. The urine of a pregnant woman contains a large amount of pregnanediol, but this had no effect on barley. Further, the diluted urine had no effect at all on wheat.
Many years later I tested the foliar application of dilute fulvic acids on soybean plants. There had already been many claims by fulvic acids vendors that such foliar applications substantially increased plant growth. I was sceptical of such claims and therefore did my own test. I was surprised to find that the fulvic acids increased the growth of soybeans by about 10%. Other ingredients including contaminants in the fulvic acids were too dilute to give such a relatively large increase in growth, so the only reasonable explanation was that fulvic acids act like a hormone, analogous to the situation described above where progesterone hormones increased barley growth.
An important question is whether fulvic acids are a general plant hormone, and research needs to be done to check this. The concentration of fulvic acids that I used for foliar application was quite low and therefore very cost effective for agriculture. In addition, other fulvic acids research also needs to be done due to the paucity of knowledge about fulvic acids, and I will give some insight into this below.
1. What are Fulvic Acids?
A cynical but true answer would be: “God only knows!”
2. What is my definition of Fulvic Acids?
A yellow-brown substance that dissolves in 0.1 M hydrochloric acid and is retained on an XAD-7 resin that has been equilibrated with 0.1 M hydrochloric acid.
3. Is there a satisfactory Fulvic Acids analysis available?
No. Manufacturers generally measure the visible light spectrum, but unless one already knows that the fulvic acids is highly purified, the test is inaccurate. Fluorescence absorbance at 400 to 600 nm with an excitation of 340 nm is far more accurate.
4. Are there Fulvic Acids standards to perform fluorescence analysis?
No. (I have developed a method to obtain pure fulvic acids according to my definition above, but have not commercialized it. Perhaps there is a 3rd party that is interested in this.)
5. How are Fulvic Acids obtained?
The most common source of fulvic acids is direct extraction from peat bogs. Lignite coal can also be slowly oxidized in air to form fulvic acids, which are then extracted by methods that are usually trade secrets. Note that contrary to manufacturer’s claims, I have never found more than trace amounts of fulvic acids in humic acids obtained from lignite coals.
6. Can Fulvic Acids be obtained from microbiological processes?
In recent years numerous manufacturers have started to market fulvic acids that are far cheaper than the traditional fulvic acids obtained from peat bogs or the oxidation of lignite coals as described above. These “fulvic acids” are by-products of microbiological processes. They are colored compounds that probably comply with the visible light absorbance as described above, and in that sense fall within the loose definition of fulvic acids. However, I have found their composition to be extremely variable and they may not necessarily be adsorbed onto an XAD-7 resin as described above.
Whether or not these by-products contain fulvic acids may not be important if the products enhance plant growth. If such enhancement occurs it would probably be through a plant hormone-type effect. I have not seen trials that demonstrate this, but concede that they may exist.
7. Can Humic Acids be converted to Fulvic Acids?
Yes. I have done very little humic/fulvic acids research in recent years, but occasionally I investigate some matters. Recently I discovered that humic acids slowly convert to fulvic acids under solvent extraction methods. First, the humic acids are dissolved in dilute alkali such as 0.1 M sodium hydroxide, and are then left in contact with solvents of decreasing polarity. Smaller and smaller fractions of the original humic acids become soluble with decreasing solvent polarity, but even a small amount is soluble in n-hexane.
8. How is the conversion of Humic Acids to Fulvic Acids explained?
I believe that neighboring carboxyl and hydroxyl groups within a humic acid molecule slowly react with each other to form a lactone or hydroxy lactone, which are cyclic esters. This esterification process then makes the molecule more hydrophobic and soluble in organic solvents. The humic acids then become fulvic acids. The more lactones that form within the molecule compared to remaining free carboxyl and hydroxyl groups, the more hydrophobic the compound is, and those molecules that dissolve in n-hexane probably contain only lactones and no carboxylic acid or hydroxyl groups at all.
In the section of this site entitled “Structure and Origin of Humic Acids and their Relationship to Kerogen, Petroleum, Bitumen and Coal”, I made the following statement from work that was done with fulvic acids in 1993 and published in 2003:
“A fulvic acid reacted with acetic anhydride/ pyridine (3:1), but not butylated, showed major IR bands for CH2, CH3, lactone, and acetate groups, and only very minor H2O bands. The spectrum is shown in Fig. 10, highlighting the lactone and acetate bands.”
This was accompanied by Figure 10:
Acetic anhydride/pyridine (3:1) is a good solvent for giving both acetate esters and lactones. The 1776 cm-1 solution IR band is due to lactone, and the 1737 cm-1 band is due to acetate. In conjunction with these data, the latest research demonstrates that fulvic acids are simply humic acids with lactone groups in place of separate carboxylic acid and hydroxyl groups.
11 July 2010
In reply to Dr. Kim H. Tan.
Hello Kim
I am very happy to hear from you again and I hope that all is well with you. I would like to give you some background information regarding my humic acid research. I have not been involved in this now for about 10 years or so, but at present I have the opportunity to do a small amount of work. I am extracting quite large amounts of humic acids from dry plant material and hope to make this information known later this year or next year under the title: “The Critical Experiment That Was Never Done.” I want to elaborate on our previous work where we showed that humic acids are formed in senescent plant matter well before this gets into the soil or near microorganisms.
When I first began humic acid research in 1986 as an organic analytical chemist I was quite excited as it was part of a large project looking at fluorescent banding in corals which is now routinely used for rainfall reconstruction and climate work. However, my excitement soon turned to disappointment as I researched the literature. The limited literature that I consulted was very inconsistent and confusing, and then I came across the then recent 1985 paper by Farmer and Pisaniello, which made me realise that I would have to start from the beginning to make any headway. I had a great librarian (Ms Inara Bush) and I must give her much of the credit for going back in history and finding all sorts of obsure manuscripts and reports. One must remember that this was in the late 1980s and early 1990s when the Internet was only beginning and it was much more difficult than today to locate information. For example, she found the first report of humic acid extraction (by Achard) in the Natural History Museum, London, but they were reluctant to try to photocopy the article because of the age of the document. She somehow managed to convince them to do it! The University of Queensland and the State Library of Victoria also provided many articles, and my ability to read some German, even some of the old style writing, was extremely useful. Another set of articles about fumaric, maleic and 4-oxo-2-butenoic acids, which were key papers describing the conversion of furfural into these acids, were in Spanish. Since I can also read some Spanish (but no more languages) I was very lucky.
After receiving hundreds of manuscripts and reports dating back to 1786 I decided that I could achieve something useful by deciding for myself what was more correct and what was less correct. The papers that inspired me were the one by Shapiro from 1957 and the one mentioned above by Farmer and Pisaniello. Especially the paper by Shapiro made me realise that I should work with organic solvents, so my main tool became solution FTIR because by default almost nothing else worked. Of course, I now also had a treasure of knowledge about the history of humic acid research and felt compelled to share it so that past errors did not have to be repeated. However, another disappointment has been the reluctance of long-time workers in the humic acid field from accepting the discoveries that I made. I have contacted most of the office-bearers worldwide from the IHSS, but with little response. Some of this lack of acceptance is understandable since few soil scientists would understand the complex organic chemistry involved, but on the whole, we need a new generation of researchers to forget the old dogmas and take a new approach. I am happy that in your recent book you have taken a much more enlightened view of the humic acid saga, and I know first hand that it has enlightened researchers in the field.
Many regards
Michael
NEW BOOK:
SECOND EDITION OF DR KIM TAN’S BOOK “HUMIC MATTER IN SOIL AND THE ENVIRONMENT:PRINCIPLES AND CONTROVERSIES” WAS PUBLISHED ON 10 JUNE 2014 BY CRC PRESS (495 pages, 69 illustrations; ISBN 9781482234459)
A READER’S COMMENTS AND HUMIC/FULVIC ACIDS IN THE DIET
27 October 2015
Recently I received an email that I would like to share with readers because some persons may benefit from the information. I have included my reply, plus I have given some further information about the presence of humic/fulvic acids in our diet.
Hello Michael:
I just started following your work on Humic Acid. Thank you so much for sharing with the world your knowledge. I know this type of selflessness came from the teachings of some wonderful parents, I know this, because I am the same way.
Before I go any further let me say to you, “my condolences to the passing of you dad in 2003”. I needed to say that to you, if not I would not be able to comfortably go further with my communication to you.
In 2003 my life changed. I had just gotten accepted to Brooklyn Law School and at the time Graduating from John Jay with a Bachelor in Forensic Psychology. However at that graduation I passed out and was rushed to the hospital. I was told by the doctor, if I had not come in, I would have been dead in three months. My TCell count was 290 and viral load was 20,000. I had HIV. How I contract it was more heart breaking than the virus itself. I am a very strong personality and fear is not a part of it at all. I am very intelligent with a great deal of common sense and reasoning deduction. I was not defeated by a virus, but knew I would find the cure.
I searched for thirteen years and in 2013 I found it in Fulvic Acid / Humic Acid. Mostly I found it in the Humic Acid Powder. The fulvic liquid was used later in time, but the journey for vitality in health came from Humic Acid. I do not know you Michael, but my spirit tells me I do. I say this because the next thing I did after realizing what it did for me, kinda makes me think of you in your same selfless way.
I monitored my blood work through my doctor, but also monitored my own personal development. After a week of taking two spoon fulls a day of the powder I had my first blood test which blew my mind. I never usually went over 500 with my TCells, but my Viral load has always been undetectable since day one of the diagnoses. This the first test I took after seven days of humic, my tcells were 897 and the viral load was still so undetectable it could not be measured.
I immediately ran out and gave it to the seniors I love so very much. They have been sick with mineral deficiencies and the acidic environment in their bodies housed so many illnesses. This I knew because I began to research the body, acidic environments and Alkaline environments. My entire life changed with Humic. One of my loved ones had Vertigo and now after one month of Humic she is driving again.
Michael the demand for the Humic Powder I have been giving away has gotten so great that I had to build an online store. I am however stuck at a juncture and would really love your advice. I am stuck with the FDA, supplemental facts. I am so baffled at this point. Can you give me some advice for the only substance of humic that Know has been given FDA clearance is Humifulvate, That’s not even US, but Europe, it went through the FDA, because it was being sold here in the US as well.
Michael whatever ideas or information you feel may help me would be humbly appreciated. I follow you on wordpress, again I love your work. I also have a blog on wordpress called; defeatingimmunedeficiencies@wordpress.com. My blog has absolutely nothing on your break down of Humic Acid. No. It is more of a personal journey with Humic Acid, how it was introduced to me, and how it began to change my life. By the way Michael, I have not been on the pharmacy medication since 2013, I no longer am required to take it, because I no longer need it.
Sorry for the long winded story, but I wanted to give you a little back ground so you would better understand How Humic Acid Powder literally changed my life and gave me life back. This mineral is a threat to the establishment, so Michael please keep that in mind as well while answering my question k.
Many Blessing to you.
Thanks So Very Much.
Nydja Wages
Reply:
Hello Nydja
Thanks for sharing such a positive experience of human emotion with me. Discoveries by themselves are of little value unless they can benefit human and animal life.
I am not sure what advice you would like me to give, so could you please clarify this. Also note that I am not up to date with humic acids and the FDA or any other regulatory body. However, after having worked in the pharmaceutical industry early in my career I am well aware that to register products for human use is not easy, and unless one has hundreds of millions of dollars to spend on safety, efficacy and toxicity testing a product can never be registered.
Regards
Information Regarding Humic/Fulvic acids in the diet:
In 1989 my colleagues and I published the first information that humic acids are found in senescent plant matter (Susic, M. & Boto, K.G. High-performance liquid chromatography determination of humic acids in environmental samples at the nano-gram level using fluorescence detection. Journal of Chromatography, 502, 443-446 (1989)). This was an important breakthrough because it showed that the old dogma of humic/fulvic acids being formed in the soil by microbiological conversion of plant lignins lacked credence. The finding has now been repeated and well documented. In my article “MORE THAN TWO CENTURIES OF HUMIC ACID RESEARCH—WHY SO LONG?” on this website under the section “THE CRITICAL EXPERIMENT THAT WAS NEVER DONE 18 February 2011”, I showed that common foods such as toasted bread and rotting pears also contain humic/fulvic acids.
From my research it appears that not only senescent plant matter, but any plant matter that contains the sugars xylose (from xylans or hemi-celluloses) or fructose (from fruits and sugar cane) when it is dried, heated, roased, toasted, fried or allowed to rot is a valuable source of humic/fulvic acids. I have not done the research but invite others to show that foods such as molasses, Panela, brown sugar, coffee, tea, sultanas, raisins, currants, toasted bread, bran, brown bread, brown rice, roasted nuts, chips and similar snacks, malt, etc. are valuable sources of humic/fulvic acids. I also believe that the underneath brown-black part of mushrooms is a good source of humic/fulvic acids.
On 6 December 2003 in my article on this site entitled “STRUCTURE AND ORIGIN OF HUMIC ACIDS AND THEIR RELATIONSHIP TO KEROGEN, BITUMEN, PETROLEUM AND COAL” I wrote that “Hopefully this research will inspire new directions in a confused field.” Although I have done minimal work in this field since then, it is inspiring to see that a new generation of researchers and people interested in the humic/fulvic acid field are making new efforts to really understand this commodity that is a critical part of life on Earth.




E8al1z Thanks for good post
Comment by johnny — 2009 @ 5:35 pm
I AM SEEKING A METHOD TO EXTRACT HUMIC & FULVIC ACID FROM LIGNITE THAT COULD BE USED ON A COMMERICAL SCALE.CAN YOU HELP ? ENJOYED YOUR JOURNEY INTO THE PAST.SEEMS THAT LIGNITE BRINGS THE DAWN OF A NEW AGE AND AN ANTIDOTE TO THE ‘SAVE THE PLANET PANIC’
GO WELL
O.B.
Comment by OWEN BEADLE — 2009 @ 11:40 am
Hello Owen
If the lignite has enough natural humic acid it can simply be extracted with sodium hydroxide or potassium hydroxide if for agricultural use. I have tested lignite materials from NZ and they have contained much humic acid. If not, the lignite needs to be oxidised with nitric acid or dilute hydrogen peroxide, or left exposed to oxygen from the air for ~1 yr or so, then extracted.
Lignites do not contain fulvic acids even though some people will claim this. Fulvic acids occur in peat bogs.
Regards
Michael
Comment by humicacid — 2009 @ 10:35 am
sir, i m student from jawaharlal nehru agricultural univesity india .sir i msearching for the method of producing humic acid from legnite for commertial use in .if u got the method plese forword it to me.i m in need of it for my project work for fulfeilment of degree.plz give me the same.thanking you.sir my email is .harishkhandal@gmail.com
Comment by harish — 2009 @ 4:25 pm
Hello Harish
Leonardite is oxidised by air, hydrogen peroxide or nitric acid. It is then extracted with either sodium hydroxide or potassium hydroxide and dried. The process is simple but can be inefficient. I have an excellent patent reference on another computer that I will send to you later.
Regards
Michael Susic
Comment by humicacid — 2009 @ 10:39 am
I want to manufacture humic acid fertilizer. Requires investor. Raw materials and logistic support no problem
Comment by Imchen Mar T — 2009 @ 7:24 pm
hallo,
i m searching for method to determine the humic acid in water, can u provide me.
Comment by nadeem iqbal — 2009 @ 9:44 pm
Hello Nadeem
There are methods for determining humic acids in water, but it depends on the concentration of the humic acids. If you have a high concentration such as in the case where you dissolve a standard humic acid in water, a gravimetric method after pH precipitation can be used. However, if you want to assay humic acids in environmental samples at low concentrations (usually ppm levels), the only method is one that we developed many years ago (published 1989) that uses High Performance Liquid Chromatography (HPLC) with fluorescence detection. If you have access to such equipment I can forward you a copy of our paper. Other researchers have made some small adjustments to our original method, but unfortunately there has not been much progress in this field.
Regards
Michael
Comment by humicacid — 2009 @ 10:13 am
Hallo Michael,
Thanks for reply.i want to work on humic acid role in some special fields.i need your help in this regards.how i can make a contect with u.
Nadeem
Comment by nadeem iqbal — 2009 @ 7:53 pm
hellow
according to your humus study every thing is knoweldge able. However, the phenolic are not explained in humic substance according to my study humic extract effect in the regeneration and elongation.
according to my experiment humus inhibit the plant elongation but increased the germination at higher concentration.
Comment by Vidya Rattan — 2009 @ 9:49 pm
Hello Vidya
Results on plants depends very much on how “humus” is defined. When humic acids are rigorously purified I have found no effect on plants. However, unpurified extracts of both humic and fulvic acids (humus) contain many ingredients in small amounts that can show hormone-type effects on plants (similar to auxins and gibberellins). I suspect that in your experiment it was these extraneous compounds that gave the results you saw. Rigorous purification of humic acids and/or tests on different plants may give quite different results.
Regards
Michael
Comment by humicacid — 2009 @ 10:21 am
thanks for information.sir i m student from the jawaharlal nehru agriculture university.sir,can u give detailed procedure’s of producing humic acid(i.e.potassium humate95% powder form etc.) from leonardite.
Comment by harish — 2009 @ 4:16 pm
I like the approach you took with this article. It isn’t every day that you find a subject so to the point and enlightening.
Comment by REBEKAH MCKAY — 2009 @ 7:48 pm
sir,
i am student of tamilnadu agricultural university,i am interested in doing research on “effect of humic acid on seed germination and vigour in maize crop”. so i am collecting related articles but i couldn’t get humic acid on seed germination articles……where can i search.pls help me………
Comment by karthika — 2009 @ 3:11 pm
Hello Karthika
Here are some references:
Tan, K.H. and V. Nopamornbodi. 1979 Effect of different levels of humic acids on nutrient content and growth of corn. Plant and Soil 51:283-387.
Tan, K.H. Humic Matter in the Soil and the Environment (Marcel Dekker, New York, USA), p. 285-291 (2003).
Guminski et al. 1977 Acta Soc. Bot. Pol. XIV1:437-448
Dormaar, J.F. 1975 Effects of humic substances on nutrient uptake…Can. J. Soil Sci. 55:111-118.
Note that I studied the germination and growth of tomato and wheat seeds in water with very pure humic acid and obtained no improvement in germination or growth. In my opinion it is impurities in humic acid samples that affect plant growth by acting like plant hormones.
Regards
Michael Susic
Comment by humicacid — 2009 @ 6:34 pm
There are methods for determining humic acids in water, but it depends on the concentration of the humic acids. If you have a high concentration such as in the case where you dissolve a standard humic acid in water, a gravimetric method after pH precipitation can be used. However, if you want to assay humic acids in environmental samples at low concentrations (usually ppm levels), the only method is one that we developed many years ago (published 1989) that uses High Performance Liquid Chromatography (HPLC) with fluorescence detection. If you have access to such equipment I can forward you a copy of our paper. Other researchers have made some small adjustments to our original method, but unfortunately there has not been much progress in this field.
+1
Comment by Bram Maikawa — 2009 @ 11:19 pm
Mike:
That was a very nice article
Comment by Dr. Kim H. Tan — 2009 @ 1:52 am
Dear Sir
It is a very very nice paper, and I love this paper so much. Is this paper published? or will this paper be pubish in the future? If so, could you give me a hand to send me the oneline adress.
Comment by Yingchen Bai — 2009 @ 6:12 pm
Dear Yingchen Bai
I have no plans to publish this paper, so I have made it freely available on the Internet.
Regards
Michael
Comment by humicacid — 2009 @ 4:04 pm
Hello Caleb
Any brown senescent plant matter contains quite large amounts of humic acids that can be extracted with NaOH or KOH. However, not all is extractable because some is strongly bound to proteins or other matter.
Soak the plant matter at least 24 hr (better 2-3 days) in 0.1 M NaOH or KOH and agitate if possible several times. Take off the solution, wash the plant matter with some water, and collect this also. Filter the solution or remove all particulate matter in some other way. Add concentrated hydrochloric or sulfuric acid very slowly (dangerous reaction!) with agitation until the pH is ~1. Filter or collect the precipitate in some other way, then wash and dry (either oven or air dry). This material is humic acid and is reasonably pure. It can be further purified by dissolving in 0.1 M NaOH or KOH and precipitating again. The solid humic acid can be redissolved in 10% KOH for agricultural use to give soluble potassium humate. The amounts are such that the final product has a pH of 10-11, and this will need to be done by trial and error. The product can be used in solution or dried. Both the solution and the dried product will contain some unreacted potassium hydroxide that on prolonged standing will react with carbon dioxide from the air to form potassium carbonate instead of hydroxide, but this is not a problem.
I recently performed fluorescence spectra on plant-derived and leonardite humic acids which I have not yet put on the website, and the spectra were almost identical, which means that the humic acids from these sources are almost identical.
Regards
Michael
Comment by humicacid — 2009 @ 3:56 pm
My company, Liquid BioTech, produces actively aerated compost teas. We use humic acid as part of our recipe. We are very interested in producing large scale quantities of plant derived humic acids. Can you send me information on this process? Also is there a difference between leonardite derived and plant derived humic acids?
Comment by Caleb Adams (Liquid BioTech) — 2009 @ 7:52 am
Dear Sir:
This is a very nice article. I love it so much that I highly recommended it to my colleagues who focus on humic substance or natural organic matter. They were so surprised at finding this paper. Unfortunately, some guy was not very good at English. Can I translate this article into Mandarin, and publish it in my personal website or a Journal in China. I think if you permit foreigners to translate it into different languages, more scientists will get benefit from it. Of course, you are the definitely author of this article.
Yours Sincerely
Yingchen Bai
Comment by Yingchen Bai — 2009 @ 12:26 am
Dear Yingchen Bai
I am glad that you and your colleagues are finding the information useful because many years of difficult research went into this project.
You have my permission to translate any of the articles into Mandarin if you state that I am the author and you are the translator. This can be for your website or a journal in China.
Regards
Michael
Comment by humicacid — 2009 @ 4:01 pm
Dear Sir
Thank you so much for your permission. It is a interesting job for me. I will translate it as soon as possible……Of course…….Once it is finished, I will send you a copy version. Yes, I will state MICHAEL SUSIC is the author of the article.
Yours
Yingchen
Comment by Yingchen Bai — 2009 @ 9:00 pm
Dear Yingchen Bai
I have added a section entitled “The Critical Experiment that was Never Done” at the end of the section “A History of Humic Acid Research” that I am sure you will find interesting. If you wish you have my permission to translate this into Mandarin.
Regards
Michael
Comment by humicacid — 2009 @ 1:05 pm
Dir Sir
I am sorry to reply your message so later. I will update the translation version ASAP. Definitely, I hope I can get your permit to translate this paper into Mandarin with Michael as the author.
Are your sure your review related to humic acid only? I think we can use humic substance instead of humic acid.
Best wishes,
Yingchen Bai.
Comment by Yingchen Bai — 2009 @ 7:20 pm
Dear Yingchen Bai
You can use the term “humic substances” instead of “humic acids” if you want to do that.
Regards
Michael
Comment by humicacid — 2009 @ 6:12 pm
Michael,
I am Nathan from Dallas Texas. I read your article. I have been studying humic acids only for a few months. I discovered a large black, waxy source of material similar to what has been named Leonardite after the late A. G. Leonard.
I have had it analyzed at two labs for humic content which resulted in 13 percent humic using what they commonly call the California test for humic.
I have noticed the confusion about the humic and fulvic acids and the inability to come up with an accurate analysis of humics and nothing certain at all with Fulvics. Of course, many have differing opinions about the existence of fulvics at all. And are they actually acids before you add the acids to test for the presence of either?
Any way, my results of the plasma test shower 2300 ppm of Aluminum and 16k ppm of iron. I would like to use the humic acid to make nutraceuticals but not possible with these high levels of AL and FE.
Do you have any way to remove these elements without destroying the beneficials in the substance?
Sincerely,
Nathan Burgess
pilotschoice@hotmail.com
Comment by Nathan — 2009 @ 10:07 am
Hello Nathan
An assay of 13% humic acid is typical for black coal deposits, whereas brown coal deposits can have 30-50%. The Al & Fe may not be bound to the humic acid per se, but could be due to clay that is contaminating the humic acid. For example, when I began studying humic acids, I found that the Aldrich standard that I was using had a huge amount of clay, and this had to be purified. Try purifying your humic acid by dissolving in 0.1 M NaOH, then letting it sit for a few days first. Decant the liquid and discard any material that settles to the bottom. Then filter the liquid through a fine filter, at least 0.4 micron or so. This solution will not contain any mineral particles. Then carefully acidify to pH 1 or so with conc HCl and collect the precipitate by filtering or centrifuging. This is humic acid. Wash it several times with distilled or deionized water and dry. Hopefully this should be free of Al & Fe. The respective K, Na or NH4 soluble salts can be prepared by reacting with K, Na or NH4 hydroxides, or you can use the insoluble humic acid “as is”.
Regards
Michael
Comment by humicacid — 2009 @ 6:59 pm
Respected sir,
I found this Review very usefull for me and thank you very much for your contribution for the emerging researchers. Sir i am just now entering in to the field of research in humic acid. I am having the commercially available humic acid with me which is in the powder form. IS THERE ANY SPECIAL PROCEDURE TO USE IT? Give me some idea how to use it.. looking foreward to your response.
Regards,
Prabodha K. Meher
Student
India
Comment by Prabodh — 2009 @ 5:44 pm
Hello Prabodha
Commercial humic acids are very impure. Purify it by dissolving in 0.1 M NaOH and passing it through a 0.2 micron filter or centrifuging it. This removes the mineral particles. Then acidify the filtrate with concentrated HCl until the pH is ~1-2. Filter this and wash several times with distilled or deionised water, and dry (air dry or heat). Repeat once or twice to give pure humic acids. Various fractions can also be obtained by dissolving the purified humic acids in solvents such as ethanol and ethyl acetate, and these fractions will have slightly different properties. Soluble salts can be obtained by reacting the pure humic acids with ammonia solution, sodium hydroxide or potassium hydroxide.
Regards
Michael
Comment by humicacid — 2009 @ 8:15 pm
Good post. I learn one thing more challenging on different blogs everyday. It would always be stimulating to learn content from other writers and apply slightly one thing from their store. I’d choose to use some with the content material on my weblog whether you don’t mind. Natually I’ll provide you with a link in your internet blog. Thanks for sharing.
Comment by mahjong — 2009 @ 11:08 am
Hello Mahjong
You can use any of the material on your blog if you provide a link.
Regards
Michael
Comment by humicacid — 2009 @ 8:17 pm
Respected Dr.Michel Susic,
i am about to mad ! what an article.
how is it possible to collect all information and make research over development. i realize after looking this article and references you must spent more then 3/4 of time in your life for research on Humic acid. am i right ?
i am a student and soon facing the real world, i love nature and natural agriculture practices. what should i do to protect our system from blind use of chemicals ?
Sincere,
Tabrez
Comment by Tabrez — 2009 @ 7:56 pm
How do you produce Humic acid from compost/plant material?
Comment by Caleb Adams — 2009 @ 1:32 pm
Hello Caleb
This is the information that I sent you some time ago. Perhaps you did not receive it, so I will send it again:
Any brown senescent plant matter contains quite large amounts of humic acids that can be extracted with NaOH or KOH. However, not all is extractable because some is strongly bound to proteins or other matter.
Soak the plant matter at least 24 hr (better 2-3 days) in 0.1 M NaOH or KOH and agitate if possible several times. Take off the solution, wash the plant matter with some water, and collect this also. Filter the solution or remove all particulate matter in some other way. Add concentrated hydrochloric or sulfuric acid very slowly (dangerous reaction!) with agitation until the pH is ~1. Filter or collect the precipitate in some other way, then wash and dry (either oven or air dry). This material is humic acid and is reasonably pure. It can be further purified by dissolving in 0.1 M NaOH or KOH and precipitating again. The solid humic acid can be redissolved in 10% KOH for agricultural use to give soluble potassium humate. The amounts are such that the final product has a pH of 10-11, and this will need to be done by trial and error. The product can be used in solution or dried. Both the solution and the dried product will contain some unreacted potassium hydroxide that on prolonged standing will react with carbon dioxide from the air to form potassium carbonate instead of hydroxide, but this is not a problem.
I recently performed fluorescence spectra on plant-derived and leonardite humic acids which I have not yet put on the website, and the spectra were almost identical, which means that the humic acids from these sources are almost identical.
Regards
Michael
Comment by humicacid — 2009 @ 5:01 pm
i need material on humic acid as am working pn humic acid extraction from low rank coal. plz snd my material on my mailing id. aabigill@yahoo.com
Comment by aabi — 2009 @ 5:40 am
Hello Aabi
This is a reply I gave recently for plant matter, and it applies to low rank coal: Any brown senescent plant matter contains quite large amounts of humic acids that can be extracted with NaOH or KOH. However, not all is extractable because some is strongly bound to proteins or other matter.
Soak the plant matter at least 24 hr (better 2-3 days) in 0.1 M NaOH or KOH and agitate if possible several times. Take off the solution, wash the plant matter with some water, and collect this also. Filter the solution or remove all particulate matter in some other way. Add concentrated hydrochloric or sulfuric acid very slowly (dangerous reaction!) with agitation until the pH is ~1. Filter or collect the precipitate in some other way, then wash and dry (either oven or air dry). This material is humic acid and is reasonably pure. It can be further purified by dissolving in 0.1 M NaOH or KOH and precipitating again. The solid humic acid can be redissolved in 10% KOH for agricultural use to give soluble potassium humate. The amounts are such that the final product has a pH of 10-11, and this will need to be done by trial and error. The product can be used in solution or dried. Both the solution and the dried product will contain some unreacted potassium hydroxide that on prolonged standing will react with carbon dioxide from the air to form potassium carbonate instead of hydroxide, but this is not a problem.
Regards
Michael
Comment by humicacid — 2009 @ 12:36 pm
Hi Michael!
Before your story picks up, Charles Darwin described “leaf mould” as the source of soil fertility, and this work inspired the Soil Association and the current orthodoxy in organic agriculture. As mould was traced to the species now called Fungi, the theory of microbial origin from lignin took root!
Dawin was thinking, of course, of Europe’s verdant deciduous woodlands, with their gorgeous autumn colours. The leaves you show are more subtropical, dry yellow to brown, and yield rather little humic acid. Have you examined the yield from deciduous leaves?
This is a point of current interest, for vegetable production in China. Soil fertility as known in soil science and field agriculture is a matter of availability of key ions, which may be supplemented by mineral fertilizers. Here the role of humus is critical, and your work is really important, especially as the cost of mineral fertilizers rises with the price of oil.
Regards
Orwin
Comment by Orwin O'Dowd — 2009 @ 1:53 pm
Hello Orwin
I have not examined leaves from deciduous woodland trees. You are correct that the yellow to brown leaves that I show are typical of Australia, which is a dry country. However, there are tropical and subtropical trees here that suddenly shed all their leaves before flowering and/or growing a new season of fresh leaves. I have examined them and they are the same. I have examined wheat which is able to grow in cold climates, and it gave the same results. I have also examined fungi that feed on dead trees, and they contain quite a large amount of humic acids, so moulds could be the same. Therefore, I believe that plants, fungi and seagrasses per se all produce humic acids as their cells die. What is required is a source of polysaccharides containing monomers of xylose, arabinose and fructose, which is quite common in nature. The conversion to humic acids is detailed at STRUCTURE AND ORIGIN OF HUMIC ACIDS AND THEIR RELATIONSHIP TO KEROGEN, BITUMEN, PETROLEUM AND COAL on the website.
Regards
Michael
Comment by humicacid — 2009 @ 12:53 pm
I introduce myself as a student of chemical engineering. I am really impressed with your article. I have two questions:
1. How can I extract humic acid coming out of slurry from biogas plant
2. Please let me know the humic acid dtermination method too
Regards,
Gaurav
Comment by Gaurav — 2009 @ 10:55 am
Hello Gaurav
Please follow the method that I gave to Aabi on 18 April 2011 under “A History of Humic Acid Research” and adapt it to your product. Humic acid is readily assayed by precipitating with acid to pH 2, washing with water at pH 2, drying and weighing.
Regards
Michael
Comment by humicacid — 2009 @ 12:31 pm
Thanks for your comment Michael. My problem is that the slurry coming out contains only 20% dry matter. Should I treat the whole slurry with KOH/NaOH first or should I filter them out?
Regards,
Gaurav
Comment by Gaurav — 2009 @ 5:36 pm
Hello Gaurav
I suggest trying both to discover which works best for you.
Comment by humicacid — 2009 @ 12:17 pm
HI Micheal am a researcher and would wish to get the procedure for extracting humic acid from coal.
Comment by wanjiru maina — 2009 @ 7:38 pm
Hello Wanjiru
This is a reply I gave recently for plant matter, and it applies to coal, especially low rank coal: Any brown senescent plant matter contains quite large amounts of humic acids that can be extracted with NaOH or KOH. However, not all is extractable because some is strongly bound to proteins or other matter.
Soak the plant matter at least 24 hr (better 2-3 days) in 0.1 M NaOH or KOH and agitate if possible several times. Take off the solution, wash the plant matter with some water, and collect this also. Filter the solution or remove all particulate matter in some other way. Add concentrated hydrochloric or sulfuric acid very slowly (dangerous reaction!) with agitation until the pH is ~1. Filter or collect the precipitate in some other way, then wash and dry (either oven or air dry). This material is humic acid and is reasonably pure. It can be further purified by dissolving in 0.1 M NaOH or KOH and precipitating again. The solid humic acid can be redissolved in 10% KOH for agricultural use to give soluble potassium humate. The amounts are such that the final product has a pH of 10-11, and this will need to be done by trial and error. The product can be used in solution or dried. Both the solution and the dried product will contain some unreacted potassium hydroxide that on prolonged standing will react with carbon dioxide from the air to form potassium carbonate instead of hydroxide, but this is not a problem.
Regards
Michael
Comment by humicacid — 2009 @ 11:28 am
Helo sir,
I am very much glade to know about your work on humic acid. sir i am working on extraction of humic acid or Humic substance of purity 70-85% from lignite. sir is there any std protocols or methods for extracion and purification. could you please provide me some related patents and methods.
thankyou sir,
Regards,
Sachin kumar
sachin.dypbbi@gmail.com
Comment by sachin kumar — 2009 @ 12:16 pm
Hello Sachin
This is a reply I gave recently for plant matter, and it applies to low rank coal: Any brown senescent plant matter contains quite large amounts of humic acids that can be extracted with NaOH or KOH. However, not all is extractable because some is strongly bound to proteins or other matter.
Soak the plant matter at least 24 hr (better 2-3 days) in 0.1 M NaOH or KOH and agitate if possible several times. Take off the solution, wash the plant matter with some water, and collect this also. Filter the solution or remove all particulate matter in some other way. Add concentrated hydrochloric or sulfuric acid very slowly (dangerous reaction!) with agitation until the pH is ~1. Filter or collect the precipitate in some other way, then wash and dry (either oven or air dry). This material is humic acid and is reasonably pure. It can be further purified by dissolving in 0.1 M NaOH or KOH and precipitating again. The solid humic acid can be redissolved in 10% KOH for agricultural use to give soluble potassium humate. The amounts are such that the final product has a pH of 10-11, and this will need to be done by trial and error. The product can be used in solution or dried. Both the solution and the dried product will contain some unreacted potassium hydroxide that on prolonged standing will react with carbon dioxide from the air to form potassium carbonate instead of hydroxide, but this is not a problem.
Since historically humic acids have been extracted by a base such as sodium hydroxide, such methods are not patented. However, numerous other extractants have been tried and these could have been patented, but I am not aware of such patents. Perhaps you could do an internet search for “humic acid extraction” and list some solvents such as aqueous potassium carbonate, phosphoric acid, acetic acid, etc.
Regards
Michael
Comment by humicacid — 2009 @ 11:33 am
Dear Sir,
I have read your artical on humic acid. Itis quite infomative and good guide for humic acid production. It is good service to numankind.
Tanking you,
M.D.Patel
Comment by manohar patel — 2009 @ 9:17 pm
Dear sir, I am very much impressed with all your work on humic acid.I have tried to extract humic acid from lignite with KOH solution. But after extracting with KOH solution, I found some fungal colony on Liquid potassium humate. So I repeat it again and same result came,So please tell me the reason for same. and also how to reduce the pH of extracting solution from 12.5 to 10?
Comment by Balram — 2009 @ 2:19 am
Hello Balram
I have found fungal colonies on potassium humate extracts when the extraction procedure took too long, such as several days, but never after one or two days. These fungi must be very virulent to grow in such a highly alkaline environment, but they only grow on the surface. What I do is just physically remove them and during the purification process they have never returned. Perhaps you can add potassium sorbate to the solution, but I doubt that it will possess sufficient efficacy against the fungi at such a high pH.
The pH of the extracting solution can be lowered from 12.5 to 10 by carefully adding dilute hydrochloric acid and monitoring the pH until it reaches 10.
Regards
Michael
Comment by humicacid — 2009 @ 11:41 am
Respected sir, myself Pranjal Handique, is a Jr. Tech. at Coal Chemistry Division in North-East Institute of Science & Technology under CSIR(Council of Scientific & Industrial Research). Can i know the method that how to use coal samples as soil conditioner in R & D status? Reply please at phandique0@gmail.com.
Comment by Pranjal Handique — 2009 @ 4:38 pm
Hello Pranjal
Low rank coal (e.g. lignite coal/brown coal) are commonly applied to agricultural soils at quite high rates (up to tonnes/ha) as soil conditioners because they contain reasonably high concentrations of humic acids. The humic acid concentrations can vary between 10-50% depending on source.
It is a bad practice to add coal dust that contains very little humic acids to soils because the fine particles are resistant to microbial breakdown and clog soil pores. These pores are essential for many processes involving micro & macroorganisms and plant roots.
Regards
Michael
Comment by humicacid — 2009 @ 11:47 am
Dear Sir,
The Article is really very nice….Thanks for making it available online….
We are working on isolation of humic acids on commercial scale and also on the agricultural importance of fulvic acids. I have found many methods including acid alkali separation and gel filteration and chromatographic techniques. Which one of these is most efficient on commercial scale??? What is the recovery percentage if we follow acid alkali separation method ??? Can refluxing decrease the processing time…..???
Swati Dhakar
Comment by Swati Dhakar — 2009 @ 4:37 pm
Hello Swati
Alkali extraction is the most economical/efficient method for commercial scale humic acid isolation. You will need to perform your own tests to determine the optimum alkali concentration, extraction time, and number of extractions. Generally, extraction efficiency increases with (a) contact time between the raw material and alkali, (b) increasing the surface area of the raw material (i.e. transform into a powder if possible), and (c) agitation (either mechanical or air). Therefore I suggest milling the raw material and extracting for 16-24 hr with agitation, and performing this process two or three times on the same material. I do not think that refluxing would be economical (heating is expensive), but you may wish to try it.
Regards
Michael
Comment by humicacid — 2009 @ 12:01 pm
Hi every one,
I have done one experiment for the extraction and purification of humic Acid from Purified Lignit. through Alkali treatment at very low concentration. and the recovery was approx 70 %, but now i want to increase the productivity, so could you all pease gives some valuable sugession s,
Thankyou,
sachin.dypbbi@gmail.com
Comment by sachin kumar — 2009 @ 6:33 pm
is humic acid produced in any soil bacteria???
Comment by Saurabh Khanduri — 2009 @ 2:04 am
Hello Saurabh
My research has shown that humic acids are produced chemically through a series of reactions from the pentose sugars xylose, arabinose, ribose and fructose (fructose can exist in the pentose form) when a living organism or part of the organism (e.g. plant leaf or alga) dies. Bacterial cell walls are composed of peptidoglycans, which do not contain these sugars, although some peptidoglycans contain ribitol, but I do not think that this will produce humic acids. Therefore, it is most unlikely that soil bacteria will produce humic acids because they do not produce them directly, and they do not contain the appropriate sugars to produce them when they die.
Regards
Michael
Comment by humicacid — 2009 @ 12:36 pm
Dear Sir I like this article. I am working as a Scientific
officer in Vasantdada Sugar Institute, Pune. Recently I am
preparing one research project on “Effect of humic acid on
growth, yield and quality of sugarcane”. We developed one
multinutrient liquid fertilizer. Content of Multimacronutrient
is NPK 8:8:8 % . so we want to add humic acid in this product.
and improve our product. Is it possible or not. please give
me some suggestion
Comment by Dr. Preeti Deshmukh — 2009 @ 9:06 pm
Hello Preeti
To add humic acids to a fertilizer is an excellent idea because humic acids are soil conditioners and improve soil characteristics and assist nutrient uptake. If you add raw humates such as lignite coal products, you can add up to several hundred kilograms per hectare; same with insoluble humic acids. However, the best results are with soluble agricultural humates such as potassium humate. Add potassium humate at 20-50 kg/ha (more will become inhibitory to some plants).
Regards
Michael
Comment by humicacid — 2009 @ 12:15 pm
yes definatly you can add Humic acid in your mix micronutrient and it will definatly works , i have lot of papers which proves efficasy of humic acid in increasing growth and yield parameters of sugercane. i Was worked for three months at VSI,Pune.
Comment by harish khandal — 2009 @ 3:15 am
people just want to read quality cintent like yours, nice
Comment by research example — 2009 @ 7:43 am
Hello Michael, my name is Anna, and we are looking some lab to analize humic acids wich was made from organical compost. Were we can do this? We are in Ecuador. thanks.
Comment by Anna — 2009 @ 2:48 am
Hello Anna
I recommend A&L Western laboratories in USA (http://www.al-labs-west.com%5B/sections/anservices/soil/fees%5D).
Regards
Michael
Comment by humicacid — 2009 @ 12:02 pm
Thanks a lot Mr Michael for sharing such valuable information!
Comment by Reve — 2009 @ 5:26 pm
respected sir i want to that how humic acid can be extracted from coal on commercial scale.
Comment by shumila — 2009 @ 10:54 pm
respected sir i want to know that how humic acid can be extracted from coal on commercial scale.
Comment by Shumila Phantam — 2009 @ 10:58 pm
hello sir, i am the student of MPUAT university, India>>>>>>sir can we extract humic acid form the waste slurry of biogas plant>>>>>>>>and if we can extract it then what is the procedure for the extraction of humic acid from the waste slurry>>>>can you tell me????
Comment by jyoti — 2009 @ 6:59 am
Hello Jyoti
Humic acids can be extracted from any material, including waste products. The process is the same as extracting humic acids from coal. Please refer to my previous answers to this question.
Regards
Michael
Comment by humicacid — 2009 @ 2:58 pm
sir can u give me some references regarding humic acid extraction process and its qualitative analysis. if u can please forword it to me. sir i need of it for my project work for fulfillment of my degree. thanks sir. my email is juhiudaipur@gmail.com
Comment by jyoti — 2009 @ 9:59 pm
Hello
The extraction and analysis is simple. Please refer to the same question that I have previously answered below.
Regards
Michael
Comment by humicacid — 2009 @ 7:31 am
sir i have 1 Q sir can i use acetone in place of NaOH/KOH for the extraction of humic acid form biogas spent slurry. thanks sir :)
Comment by jyoti sharma — 2009 @ 5:28 pm
Hello Jyoti
Acetone extracts very little humic acid. The alkaki hydroxides are far superior. Other organic solvent such as ethanol, n-butanol and ethyl acetate also extract some humic acid, but again, it is very small compared to the alkali hydroxides.
Regards
Michael
Comment by humicacid — 2009 @ 2:54 pm
thank you sir for this useful information. sir can u tell me what is the actual method for quality analysis of humic acid. at present we use spectrophotometer method(E4/E6 ratio, E4& E6 are absorption at 465nm & 665nm respectively) for qualitative analysis.
Comment by jyoti sharma — 2009 @ 4:15 pm
Hello Jyoti
The standard method for humic acid analysis is ISO 5073, which is a simple gravimetric method. Absorption (E4/E6) ratios can be useful if your humic acid is relatively pure because the method is rapid and simple.
Regards
Michael
Comment by humicacid — 2009 @ 11:54 am
kindly provide me information on the isolation of humic substances from cocoa leaf and soil from cocoa plantation
Comment by azeez musibau oyeleke — 2009 @ 2:56 am
Hello Azeez
Please refer to previous similar questions.
Regards
Michael
Comment by humicacid — 2009 @ 2:52 pm
Hi Michael,
Can you plz let me know why is humic acid not in use commercially yet. What are the main hinderances or negatives that are keeping the big agro and fertilizers companies to venture into this product? if possible plz provid eme your contact details. would be really grateful to you.
Thanks
Comment by shahzad — 2009 @ 6:09 pm
Hello Shahzad
Humic acid is actually being marketed commercially, but only by small companies. Humic acid is not a fertilizer, but a soil conditioner that improves soil health and crop sustainability. Much more dramatic crop responses in the short term can be achieved by using fertilizers, but soil health is compromised and sustainability is reduced. Large companies are driven by short-term profit margins and conventional fertilizers will give far better returns than humic acid. Unfortunately, many of the small companies make unsubstantiated and often ridiculous claims about humic (and fulvic) acids, which gives these products a poor image when the supposed results do not eventuate.
Some countries such as India and Pakistan that have discovered huge reserves of lignite coal (which contains massive amounts of humic acid) are putting much effort into humic acid research so that this material can be utilized for agriculture. New Zealand has large reserves, but unfortunately out of ignorance, it is going to be used as a fuel to generate electricity. The efficiency as a fuel is very poor because humic acid contains a high proportion of oxygen, and the lignite raw material has a high water content.
Hopefully positive research results in the future will mean that humic acid will be utilized much more for the long term benefit of agriculture.
Regards
Michael
Comment by humicacid — 2009 @ 5:36 pm
dear Sir,
I am regularly reading this blog and everytime i got valuable information about humic acid. Thank you so much for your valuable suggestion.
I want to ask you that if humic acid (12% w/w) can be used as a wetting agent aslo ? or if not then how can we make solution of humic acid that it also work as a sticking agent or wetting agent.please give me your valuable suggetion. Thank you..
Comment by Balram — 2009 @ 3:28 pm
Hello Balram
Humic acids are part of oil drilling muds and act as an inexpensive wetting agent. I am not aware that soluble humic acid solutions are used as sticking or wetting agents. Since soluble humic acid solutions in agriculture are usually potassium humate, which is very soluble, I doubt that they would work as a sticking agent. However, they could be useful as a wetting agent. The drawback to this would be the poor compatibility of humic acids with other agricultural products, so a better choice could be fulvic acid, but it is more expensive.
Regards
Michael
Comment by humicacid — 2009 @ 10:07 am
We are using lignite coal for making liquid humic acid with alkali method and making 12 % w/w….But we tried this liquid humic acid but it is not as good as commercial wetting agent to break the bond of water…. so what we can add in this liquid humic acid to work it as a sticking or wetting agent….
Comment by Balram — 2009 @ 3:07 pm
Hello Balram
Humic acids solutions are much inferior to commercial wetting agents, and there is no way that the humic acids can be modified to improve their wetting capacity. Perhaps you can add the ingredients of wetting agents to humic acids solutions, but this would need to be done in an R&D project. Wetting agents contain surfactants such as the Huntsman range of Teric and Ecoteric products, and stickers contain oils such as canola oil. A wetter/sticker may contain both the surfactants and oils.
Regards
Michael
Comment by humicacid — 2009 @ 11:21 am
u can add any non ionic emulsifier like nonyl phenol ethoxylate 9.5 mol 5%
Comment by harish — 2009 @ 4:22 am
Dear Harish sir,
Thank you . would this nonyl phenol ethoxylate 9.5 mol 5 % will really work with humic acid to work as a wetting agent/?
Comment by balram — 2009 @ 3:55 pm
Hi Michael,
Excellent article, knowledgeable and informative.
See my very recent articles in my personal web site http://www.drkhtan.net16.net/
1. The carbohydrate connections in fulvic acid chemistry
2. The new look and nanotube concept of humic acids
3. Humic acid nanotube membranes as revealed by scanning electron microscopy
Kim
Comment by Dr. Kim H. Tan Professor Emweritus — 2009 @ 2:44 am
Hello Kim
It’s great to hear from you again, and I wish you all the best for the second edition of your book “Humic Matter in the Environment. Principles and Controversies.”
I have read your recent articles, and am intrigued by your finding that fulvic acids contain more carboxyl than humic acids. I found the opposite, but my fulvic acid samples were highly refined and then derivatized, so we may have been looking at different entities. One thing that has been missed in humic/fulvic acid research is the conjugate chelate carbonyl group (https://humicacid.wordpress.com/structure-and-origin-of-humic-acids-and-their-relationship-to-kerogen-bitumen-petroleum-and-coal/ Figure 8). This group changes with the environment and has pseudo aromatic structure and properties. I believe it is this group that has made humic acid research so difficult because it changes the nature of the molecule (due to keto-enol tautomerism) according to the environment (e.g. water, solvents), so the structure of humic acids changes with what one does to them.
I am fascinated by your electron microscope work, and it certainly looks similar to nanotube structures, although that is out of my realm of expertise.
Regards
Michael
Comment by humicacid — 2009 @ 3:10 pm
Hello Micheal,
Its an excellent, informative and influential article. Thanks a lot for your nice knowledgeable papers. I am from Bangladesh, doing job as a product manager of an agrobased company, Global agrovet limited. We are importing humic acid from China and marketing in the country. The product is being popular in to the farmers day by day. Our Chinese Principal Company claimed that fulvic acid is also within it and originated from Leonardite. Is it possible? Would you please provide me some better sources of humic acid?
Comment by azam khan — 2009 @ 6:56 pm
Hello Azam
I doubt that your humic acids contain fulvic acids. I have examined a number of commercial humic acids samples and none contained fulvic acids. However, I do not think that this is deliberately misleading. Humic acids contain compounds that are yellow in solution like fulvic acids, but are not fulvic acids. The problem is that the most common fulvic acids assays simply measure the visible absorption in the yellow region, so any yellowish compound will give a response, and this is quoted as “fulvic acid” when in fact it is probably some carotene-type compound. Fulvic acids are usually obtained from peat bogs, but some companies appear to obtain them from Leonardite. Much cheaper fulvic acids are now being produced by microbiological methods. I have tested them and they appear to be similar to those obtained from peat bogs, although there is much controversy between manufacturers on this point. I recommend using them because they are far less expensive. An Internet search will give manufacturers (most from China).
Many unsubstantiated claims are made about humic and fulvic acids because knowledge of their structure and properties is poor, and some unethical manufacturers and distributors take advantage of this. Further, it is difficult to compare products from different manufacturers for the same reason. Therefore, I am unable to recommend any manufacturers or distributors. I suggest obtaining samples and then sending them for assays to A & L Western Laboratories, USA (http://www.al-labs-west.com/sections/anservices/soil/fees), and comparing costs.
Regards
Michael
Comment by humicacid — 2009 @ 1:37 pm
i am a student of chemical engineering,i want a process flow diagram for the production of humic acid from leonardite ..could you please help me out???
Comment by Amna — 2009 @ 5:02 pm
Hello Amna
Leonardite can be extracted “as is” or first oxidized. If it is oxidized, it is left in the open air for 12 months or so, or nitric acid is added for a faster oxidation. It is then extracted with a slight excess of sodium or potassium hydroxide. The extraction can be repeated for a better yield. The resulting solution is dried, and the pH should be about 10 and not more than 11.4. This is soluble sodium humate or potassium humate. To obtain humic acid the soluble humate is neutralized with acid such as sulfuric acid to a low pH (~1 is ideal), and the precipated humic acid is recovered by filtering or centrifuging. The humic acid can be purified by dissolution in sodium or potassium hydroxide, filtering at 0.2-0.4 microns to remove fine particles that strongly adhere to humic acid, and precipitating again with acid.
Regards
Michael
Comment by humicacid — 2009 @ 10:31 am
Dear Sir,
we have high sulphur tertiary coal which damages the soil in and around the coal mining areas. It is learnt that coal can the hmic beontains humic acid. claimation as well as an the humic acid be used for soil reclaimation as well as soil conditioner ? We would like the technology for manufacturing the soil conditioner for use in recalimation as well as manure.
Comment by T. Mar. Imchen. Geologist. Directorate of Geology and Mining, Nagaland, Dimapur, India — 2009 @ 11:46 pm
Dear Sir/Madam
Humic acid contents in coals can vary from around 70% to zero, depending on the type of coal. The lignite brown coals have the highest levels, and black coals have much lower levels. Please refer to previous enquiries about how to extract humic acids from coals.
Humic acids act as soil conditioners because they combine with many metals and make them more readily available to plants. They also alter soil structure to give aggregates through which air and water percolate better, i.e. soil without humic acids becomes much like concrete. These are great advantages for plants. However, they are not fertilizers and they do not have miraculous properties to restore abused soil. I am not aware that they can remedy soils severely damaged by sulfur from coal. Once soils are damaged the source of damage must first be removed, and then it can take decades to restore the soil. A perfect example of this is Queenstown in Tasmania, Australia. During the 1800s the trees were cut down and used in furnaces for smelting metals from ores found abundantly in the region. This process produced large amounts of sulfur, and the landscape in and around this town came to look like a scene on the Moon because almost nothing will grow there. I hope that the problem in your area can be remedied.
Regards
Michael
Comment by humicacid — 2009 @ 10:43 am
Hi Mr. Michael
I´m a student from Mexico, and I´m trying to research the whole process to activate leonardite with NaOH or KOH, but I can´t find a research or articule, with the specific information, Do you have the method or the process to do so, I mean the whole process with details and stuff to activate leonardite to do commercially sales, I appreciate very much if you can help me with this, thank you in advance for your attention, please send the information to my e-mail jrpala79@hotmail.com
Comment by Jose Palafox — 2009 @ 1:17 pm
Hello Jose
Please refer to my previous replies on how to extract humic acids from coals, soils, etc. It is quite simple.
Regards
Michael
Comment by humicacid — 2009 @ 10:46 am
Dear Sir
I read your previous replies, but I´m sorry, I don´t really understand the whole process and the procedure to do it on big scales, Do you have a link or articule that you can send it to me, i´ll apreciate if you can do so , thank you, my e-mail is jrpala79@hotmail.com
Comment by Jose Palafox — 2009 @ 1:42 pm
Hello Jose
I am not aware of a link or article specific to your needs. First you would need to know how much humic acid your coal deposit contains. I recommend sending samples to A&L Western laboratories in USA (http://www.al-labs-west.com%5B/sections/anservices/soil/fees%5D). Lignite coals can contain up to 60-70% humic acids. In this case the humic acids are extracted directly with sodium or potassium hydroxide, perhaps 1 M. Potassium hydroxide is used for agricultural products. For materials with low humic acid content the material needs to be crushed and allowed to stand in air for 12 months or treated with nitric acid for oxidation, then extracted with hydroxide. The potassium or sodium hydroxide extracts are dried to give soluble potassium or sodium humate, respectively. Humic acids (insoluble) can be precipitated by adding an acid such as sulfuric acid to pH 1, filtering or centrifuging, then drying. The exact procedures depend on the amount of humic acids present in your material, and laboratory tests need to be done first on the actual material to determine the best approach and concentrations of hydroxide, drying methods, etc.
Regards
Michael
Comment by humicacid — 2009 @ 7:30 pm
Hello Sir,
I’m trying to analyzed commercial available humic acid sample used for agriculture purpose [Solid as well as in liquid form]. I tried to find protocol for that….On trial basis I used 1 gm/1 ml sample and extract it with extraction solution containing NaOH, DTPA and ethanol by keeping on shaker for 1 hour.then i centrifuged it and used supernatant for spectro analysis.I used pure humic acid sample for plotting standard graph.But i got 25% humic acid in liquid sample [label claim is 15 %] and 75 % in solid sample.which is crystalline in nature.This assay is right or wrong?Can you suggest any modification for accurate testing ?And also suggest how to calculate soluble K-humate.?I also want protocol for fulvic acid estimation from commercial sample.Sir, I’m waiting for your positive response.
Thanks
Bhushan Brahmankar
Comment by Bhushan Brahmankar — 2009 @ 9:19 pm
Hello Bhushan
For fulvic acid there is not a good standard assay method available. The most common assay method is to simply measure absorbance at 400-500 nm against a standard fulvic acid. However, if there are colored compounds in the material they will interfere. A reasonably accurate assay is obtained if the commercial sample is fairly pure. If the commercial sample is not very pure an erroneous result will be obtained. A more accurate method is to pass the fulvic acid through an XAD-8 resin (expensive) column at pH ~1 and elute with base or even better with ethanol/ammonium hydroxide (4:1). The exact method would need to be developed first in a competent laboratory.
For potassium humate, dissolve in water and filter (for very accurate analysis the filter must be less than 0.4 microns because there are tiny clay particles that will combine with humic acid and precipitate with it in the next step). Then an acid such as sulfuric acid is added to the filtered solution to precipitate humic acid, which is then filtered or centrifuged out, dried and weighed. For accurate results at least 1 g of dry humic acid should be obtained. From the result the amount of potassium hydroxide in the original sample (either solid or liquid) can then be calculated.
For humic acid, the material is first dissolved in a slight excess of sodium hydroxide, and filtered (for very accurate analysis the filter must be less than 0.4 microns because there are tiny clay particles that will combine with humic acid and precipitate with it in the next step). Next precipitate the humic acid with an acid such as sulfuric acid, filter or centrifuge, dry and weigh. Try to obtain at least 1 g of dried humic acid, and from the weight calculate the amount of humic acid in the original sample.
Do not use spectrophotometric methods because there could be interferring components. This would probably explain why you obtained 25% for a label claim of 15% in the liquid. Commercial solid humic acids commonly only contain 75% or less of humic acid. They also contain water (10-15%) and insoluble coal dust (~20-30%), so your result for the solid humic acid is probably correct.
Regards
Michael
Comment by humicacid — 2009 @ 7:54 pm
dear sir, approximatly what is cost per lit, some dealer sales per lit 750=00 and 500=00 . 350=00 indian rupees . really what is price per lit. it is save to farmers, please mail me sir.
thanking yours
nandi, Email; nandimbr@gmail.com
Comment by nandimbr — 2009 @ 3:40 am
Hello Nandimbr
Humic acids, soluble potassium humates and fulvic acids are sold both as solids and liquids, with the liquids being more expensive due to manufacturing costs and possibly larger profit margins. Humic acids can be purchased for several dollars (Aust) per kilo, but the liquids only contain 10-20% humic acids so are relatively more expensive at a similar price. Fulvic acids previously were very expensive, retailing around $20-25 (Aust) per kg. However, new processes have been developed where they are produced microbiologically, or are side products of these processes, and they are around $5 (Aust) per kg. Manufacturers disagree on whether these newly produced fulvic acids are the same as the previous ones that were extracted from peat or coal beds, but in my opinion they are similar enough to warrant use in agriculture at such a reasonable price. However, one needs to be wary because I have seen some samples that contain almost no fulvic acids, but are an unknown mixture claiming to be fulvic acids.
Regards
Michael
Comment by humicacid — 2009 @ 10:57 am
thank you very much sir.many many thanks for your replay.
Comment by nandimbr — 2009 @ 12:40 am
sir i want to ask about the effect of humic acid in tissue culture and the influence of dose on herbaceous plants because I do not get literary, thank you for your concern sir . . . talgiest_91@yahoo.co.id
Comment by Harviest — 2009 @ 8:08 am
Hello Harviest
I am unable to provide information regarding tissue culture. However, humic acid is not a fertilizer, but it can alter cell processes such as mineral transport.
The influence of dose on herbaceous plants depends on the form of humic acid. Commercial humic acid is often sold as potassium humate, which is either totally or almost totally soluble. This can be applied at about 20-50 kg/hectare. If the humic acid is in the acid form, it has very poor solubility, and is used for long-term soil conditioning. In the short-term it will have little effect on plants and can be added at hundreds of kg per hectare.
Regards
Michael
Comment by humicacid — 2009 @ 2:42 pm
I found this very interesting, I am fifth generation leaching what we always called mineral water. We leach the ore using water. It is said to contain high fulvic acid content as well as high microbial count. We always thought the microbes were responsible for the acid formation. Hope I can follow this new work being done
.
Comment by Alan Eason — 2009 @ 11:05 am
I write this e-main in 2013, so it was 4-5 I did not knew The New Emerging Sciences of about Humic Acid. Wow ….. Though the world will now never afraid about the loose of this important organic substances. In 1998-2001 I and my colleague of soil sci Lab. of Agric Fac. Jenderal Soedirman Univ, Purwokerto, Indonesia; we study of the content of humic acid in the 0-25/30 cm of soil surfaces just at/of southern slope of Slamet mount near my work place. I was described down that but only in small research report. Now I am interesting again and will begin my research work on humic substances coming from plant especially water fern namely Azolla. This plant is easily to be decomposed and resulted brown to dark brown color in only 3-7 days. It will become black color in 30-35 days. Least but not last, I will also interest on how with other plant material whether it is still on that plant or lay on the soil. What is your opinion?
Comment by Purwandaru Widyasunu — 2009 @ 3:52 pm
Hello Purwandaru
When plant matter dies and becomes a yellow/brown/black color, it contains humic acids. Allow your plant matter to dry. It does not matter how you dry it, and leaving it on the soil is OK. However, do not bury it in the soil or cover it with soil because the soil will contaminate the extraction process. Also, be aware that not all the humic acid can be extracted from plant matter, even with alkali hydroxides, because it seems to stick very strongly to proteins or other matter.
Regards
Michael
Comment by humicacid — 2009 @ 2:52 pm
Sir/Madam
i need know the formula (step by step working) how can we make humic acid with lignite and potassium hydroxide as am working on humic acid extraction.How much heat , what the timeing blending process etc plz snd me material on my mailing id. khurramahmed.syed@gmail.com
Comment by khurram ahmed syed — 2009 @ 10:11 am
Hello Khurram
Please refer to previous answers to similar questions for some details. Heat is not necessary. At ambient temperature allow to stand for 24 hrs without blending, or several hours with blending. Two or three extractions can be useful. I suggest that you conduct your own experiments to find the most cost effective method since heating and blending can be expensive. Simply extract, dilute and compare the color of your extractions to determine how much you have extracted and at what cost.
Regards
Michael
Comment by humicacid — 2009 @ 9:29 am
sir i want to know about the current area of research in organic matter dynamics
Comment by priyal pandey — 2009 @ 3:26 am
Hello Priyal
I suggest that you consult Dr Kim Tan’s book:
“HUMIC MATTER IN SOIL AND THE ENVIRONMENT:PRINCIPLES AND CONTROVERSIES” 1st or 2nd edition. The second edition was published on 10 June 2014 by CRC PRESS (495 pages, 69 illustrations; ISBN 9781482234459).
Regards
Michael
Comment by humicacid — 2009 @ 12:18 pm
Hello Sir,
Your blog is quiet informative. Thanks for sharing valuable information on Humic acids.
Please let me know how to differentiate coal dust and Humic Acid.
Thanks,
Sandhya
Comment by Sandhya — 2009 @ 2:30 am
Hello Sandhya
Humic acids are soluble in alkalis such as sodium hydroxide, whereas coal dust is insoluble and can be filtered out. The humic acid can then be precipitated by adding excess acid such as hydrochloric acid and will not contain coal dust.
Regards
Michael
Comment by humicacid — 2009 @ 10:29 am
Thank you very much!!
Comment by sandhya — 2009 @ 3:23 pm
I like to know if Humic acid in raw can sustain water for longer time, I like to use Humic acid for area has shortage of water and to use water once a week
or twice a month. and if you have study show that to send me at email: ahcusa@gmail.com . also if your statement yes where we cna buy Hmic acid in blk
to be shipped to country out of USA. Please advise
DS
Comment by Humic Acid in RAw — 2009 @ 12:14 am
Dear Sir/Madam
Probably the most important role of humic acids is to give a good structure to soils. Humic acids link/complex to almost everything, including components of soil such as clay. They are one of the few compounds known that can complex with sodium, potassium and ammonium. In soil they therefore form aggregates which then creates spaces between the aggregates and allows better gas exchange (especially oxygen and carbon dioxide) and water penetration. Soils poor in humic acids are hard and do not allow good water penetration. If your soil does not already have a high humic acid content (i.e. is not dark and soft), the addition of raw humic acid will help to retain water longer, but you will need to perform some trials on how much humic acid to add and for how much longer water will be retained. However, too much humic acid in the soil (usually >>5% or so) may be detrimental to plant growth.
Humic acid is sold by many commercial suppliers from around the world, including the USA. An Internet search will give you many suppliers and I suggest that you choose one that best fits your needs. Please be aware that there is much confusion about the exact structure and properties of humic acids, so it is not uncommon for suppliers to make unreasonable claims about their products. Also be aware that most suppliers provide soluble humic acids (potassium salts), and these soluble salts can be washed out of the soil fairly quickly by rain or irrigation. For your purpose a raw, non-soluble humic acid would be required.
Regards
Michael
Comment by humicacid — 2009 @ 5:54 am
Can someone here share how to make humic acid from lignite? Please!
Comment by nambiar@cropex.in — 2009 @ 11:53 am
Dear Sir/Madam
Please refer to my previous answers to questions about extracting humic acids from soils and lignite coals. The processes for soils and lignite coals are the same. However, soils contain only several per cent humic acids, whereas lignite coals may contain 30-70% humic acids.
Regards
Michael
Comment by humicacid — 2009 @ 4:39 am
What chemicals interfere with the effects of humic acid on HIV?
Comment by Bret Harper — 2009 @ 8:40 pm
Hello Bret
The effects of humic acids on human health and well-being are poorly understood and have not been confirmed by clinical trials, although there is good anecdotal evidence and preliminary research. The same is true for animal health. Therefore, I am not able to provide any insights on what other chemicals or substances may interfere with humic acids when consumed.
For the benefit of the public, I would like to provide further information about the use of humic acids for human and animal health. In the 1930s there were obscure Russian reports that humic acids had a positive influence on human health and wellbeing, but this information was not widely available. After this time humic acids were thought to cause Blackfoot disease, especially in China, and this probably had a serious negative influence regarding the use of humic acids for human and animal consumption. However, in recent years there has been anecdotal evidence and preliminary research that has demonstrated that humic acids may have benefits for human and animal health. However, this is what Natural Medicines (http://naturaldatabase.therapeuticresearch.com/(X(1)S(n52pvr45bmwzz3552yhvrs45))/nd/PrintVersion.aspx?id=1129&AspxAutoDetectCookieSupport=1) state on their website: “Advise patients with autoimmune diseases such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), or others to avoid or use humic acid with caution.”
Humic acids for human consumption are not widely available and are expensive compared to humic acids for agriculture. Therefore, only humic acids fit for human consumption should be taken, and under no circumstances should other grades of humic acids be consumed because they may contain toxic metals, dangerous microorganisms and poisonous chemicals and substances.
Regards
Michael
Comment by humicacid — 2009 @ 4:09 am
hello friends it’s a very good information about acids for agriculture.
Comment by mybreakstyle — 2009 @ 7:04 pm
when humic acid is absorpted in a plant,how does it metabolize?
Comment by Max.G — 2009 @ 7:20 pm
Hello Max.G
Humic acids are not absorbed by plants. Humic acids are large molecules, and they bind to many substances, which prevents them from entering plant root systems.
Humic acids are not nutrients or fertilizers, but their principal role is to condition soils for optimum plant growth. They do this by binding to minerals and forming aggregates, that in turn allow good gas exchange, water flow and root penetration in the soil. An excellent course regarding this can be purchased at:http://www.biostim.com.au/biostim-certificate-in-sustainable-agriculture.html.
Humic acids may assist in the transport of minerals to plant roots, but are subsequently not absorbed by the plants. They are intractable in the sense that not even microbes can digest or assimilate them, and for this reason there are huge coal and oil fields on earth.
Many commercially-available humic acid products are actually soluble potassium humate. The plant is able to absorb the potassium, but not the humate.
Regards
Michael
Comment by humicacid — 2009 @ 9:34 am
thanks for your answer。
if Humic acids are not absorbed by plants,how to explain the good effect of spraying soluble potassium humate on plant?
ps:i am a chinese,not so well-executed in English language.T_T!
Comment by Max.G — 2009 @ 1:37 pm
Hello Max
My previous reply was specifically about root system absorption of humic acids.
I am aware that spraying soluble fulvic acids on plants causes growth increases that can be explained by the presence of hormones in the fulvic acid product, or perhaps a hormone-like activity of fulvic acid itself.
However, just like the plant root system, plant leaves will not absorb humic acids. I am not aware that scientific studies have shown increased growth due to humic acids sprayed onto plant leaves. If there is evidence that foliar spraying of soluble potassium humates increases plant growth, this could be due to the potassium, not the humic acids. Potassium is known to be absorbed by some plants through their leaves.
Regards
Michael
Comment by humicacid — 2009 @ 2:55 pm
thank you,can i understand like this?
fulvic acidplant can be absorbed by plant roots or leaves,but humic acid cannot.
Comment by Max.G — 2009 @ 1:37 pm
Hello Max
I am not aware of any research that shows whether humic acid or fulvic acid can or cannot be absorbed by plant roots or leaves. From my own research I only have anecdotal evidence that humic acids are not mobile enough to be absorbed by plant roots or leaves. I have not done any work with fulvic acid absorption by roots and I am not aware of any such work. Fulvic acids may be absorbed by plant leaves because they can cause substantial growth increases. However, since the structure of fulvic acid is not known and there are no analytical methods for fulvic acids in plant leaves, this cannot be verified.
Regards
Michael
Comment by humicacid — 2009 @ 4:50 pm
Hello Max
There is no research that demonstrates whether humic or fulvic acids are absorbed by plant roots or leaves. From my own experience humic acids are not mobile enough to be absorbed by plants and simply stick to the roots and leaves.
Fulvic acids may be absorbed by plant leaves because they cause growth responses in some plants. However, since the structure of fulvic acid is not known and there are no assays for fulvic acids in plants, leaf absorption cannot be verified.
Please note that humic acids (and possibly fulvic acids) are created in plants, especially when they die. In my research I always found humic acids in plant leaves, so whether plants absorb or do not absorb humic acids (and possibly fulvic acids) may not have any meaning.
Regards
Michael
Comment by humicacid — 2009 @ 5:03 pm
thank u, can you tell me how humic acid was metabolized when it was absorpted in a plant?
Comment by Max.G — 2009 @ 7:34 pm
Is the process of manufacturing of Humic acid from Leonardite is economically viable ?
Comment by Yogesh D. Kakkad — 2009 @ 9:44 pm
Hello Yogesh
Soluble potassium humates derived from humic acids in Leonardite coals are marketed commercially by many manufacturers, so one would have to assume that it is economically viable.
Insoluble humic acids can be regenerated from the soluble potassium humates by adding acids, but these products are manufactured much more rarely, and I cannot vouch for the economic viability.
Less commonly, the unprocessed Leonardite coals are also marketed, but transport costs can be high.
Regards
Michael
Comment by humicacid — 2009 @ 2:47 pm
Hello Michael,
I would like to know what is the best method for comparing the 2 types of humic acid (granules and liquid) interms of solubility and acidifying after adding citric acid and sugar acid to each humic acid.
Thanks
Kima
Comment by Kima Omara — 2009 @ 6:48 pm
Hello Kima
If by liquid humic acid you mean the potassium humates, which is simply the granules dissolved in water, when acids such as citric acid are added to the liquid or dissolved granules, an insoluble precipitate of humic acid results.
If by liquid humic acids you mean a suspension of humic acids in water, the addition of an acid such as citric acid will have no effect. If by granules you mean insoluble humic acids and not the soluble potassium humates, the addition of acids such as citric acid also will have no effect.
Regards
Michael
Comment by humicacid — 2009 @ 9:54 am
You have done alot of work. keep it up
Comment by Malo Mahwash Amos — 2009 @ 5:34 am
Hellp Michel, I am a student. i want to know tht how can i make humic acid balls from humic acid powder as u knw humic acid powder is totally soluble in water so how can i make balls?
Comment by Kiran Solanki — 2009 @ 12:19 am
Hi there,
I have read all the comments regarding humic and fulvic acids. I am doing an experiment where I used leonardite coal to extract fulvic acid. I occured only one problem: After I crushed the coal into smaller pieces and diluted it with water and KOH my mixture starts to become a slurry which makes it very difficult to centrifuge. Also, when I leave the solution for a week the pH tends to drop and more KOH must be added to keep the pH above 12. I saw in one of your comments that the pH must not be more than 11.4. Could you tell me why? Also, could you please advice me on how to prevent this slurry formation without adding more water or KOH?
Comment by Annusca — 2009 @ 7:05 pm
Hello Annushca
1. I have never found a Leonardite coal sample that contains fulvic acids. From my experience they only contain humic acids.
2. You obtain a slurry because humic acid molecules “stick” together. The only way to overcome this is to dilute with water or add more KOH, which means that you must centrifuge large volumes, which is a time-consuming process. I suggest that you dilute the slurry and filter through a Whatman no. 4 filter paper or similar. After that you may still need to centrifuge but it will be easier because the sample will be much cleaner.
3. The pH decreases on standing because the KOH reacts with CO2 from the air. This happens in general when any alkali is allowed to stand in contact with air.
4. pH 11.4 is a cutoff point for dangerous goods. If affects transport regulations but has no relevance to your work.
Regards
Michael
Comment by humicacid — 2009 @ 3:22 pm
I still got a small yield. I used the same method as you explained before and I even tried to heat the process. Is there a standard to apply in order to calculate the yields? I have read the Lamar method but I cannot obtain the ash content.
Comment by Annusca — 2009 @ 8:43 pm
Hello Annushca
A method to analyse fulvic acid has been recently developed and accepted by industry
(https://www.minerallogic.com/testing-method), but you require enough material to be weighed. Previously industry had a very poor method by which the adsorption by visible light (450 – 500 nm) was used with an arbitrary equation.
Regards
Michael
Comment by humicacid — 2009 @ 3:24 pm
Good day,
I have done tests on my humic acid samples and my samples all have a very low total carbon content. What is the reason for the low values in terms of literature values?
Comment by Annusca — 2009 @ 10:18 pm
Thank you verymuch for ur valuable informations regards Dr NARAYANRAO LS senior Agrinomist United Scientific solutions
Comment by Dr NARAYANRAO LS — 2009 @ 2:29 pm
Thanks to Publisher. Comprehensive an article. http://www.bat-guano.net/humic-acid.html
Comment by Ürgüb Agriculture Mining — 2009 @ 8:44 am
Thanks for this helpful sharing. https://www.urgubleonardit.com/ is a detailed website but requires online translation from Turkish to English or others.
Comment by Ürgüb Agriculture Mining — 2009 @ 11:11 pm